Research Article
Synthesis of some new Schiff bases of Pharmaceutical Interest

Ajay Kumar, Shweta Verma, Arun K Mishra and Sushil Kumar*
Faculty of Pharmacy, IFTM University, Moradabad-244102(U.P), India
*Address for Correspondence: Dr. Sushil Kumar, IFTM University, Moradabad-244102 (U.P.) India, Email: sushilmpharm@rediffmail.com; drsushiliftm@gmail.com
Dates: Submitted: 17 August 2017; Approved: 25 September 2017; Published: 26 September 2017
How to cite this article: Kumar A, Verma S, Mishra AK, Kumar S. Synthesis of some new Schiff bases of Pharmaceutical Interest. Ann Adv Chem. 2017; 1: 053-056. DOI: 10.29328/journal.aac.1001006
Copyright License: © 2017 Kumar A, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Schiff base; Diphenylamine derivative; Antibacterial activity; Amines
Abstract
A series of Schiff bases of diphenylamine derivatives have been synthesized and evaluated in vitro for their antibacterial activity against pathogenic both Gram-positive bacteria B. subtilis and Gram-negative bacteria E. coli using ciprofloxacin as standard drug at conc. of 50 µg/ml and 100 µg/ml. The structures of these compounds were established on the basis of IR and 1H-NMR spectral analysis. The compound (3d) displayed potent antibacterial activity against Bacillus subtilis (17 and 15mm) and Escherichia coli (19 and 17mm) by disc diffusion method.
Introduction
The major classes of almost all antibiotics are encountering resistance in clinical applications [1,2]. Searching for novel antimicrobial agents is in demand in the global market to counter life-threatening infections. In order to overcome this rapid development of drug resistance, new scaffolds need to be developed, preferably consisting of chemical characteristics that clearly differ from those of existing agents. The compounds with the structure of -C=N- (azomethine group) are known as Schiff base, which are usually synthesized from the condensation of primary amines and active carbonyl groups. Schiff base have been of great interest in medicinal chemistry for their role as potent antimicrobial, antitubercular, anticancer, antiviral, antimalarial, antibacterial and antifungal agents [3-10]. Therefore, Schiff bases are important compounds due to their wide range of biological activities and their industrial applications. They also serve as a back bone for the synthesis of various heterocyclic compounds [11]. In view of the wide interest in the biological activities and profile of Schiff bases, we described herein the synthesis, characterization and antibacterial activity of some new Schiff bases of diphenylamine derivatives.
Materials and Methods
All reagents were purchased from CDH of commercial quality and were used without further purification. Melting points of the synthesized compounds were determined by open capillary method and are uncorrected. The IR spectra of synthesized compounds were recorded in potassium bromide discs on Schimadzu FTIR Spectrophotometer 8300. The 1H NMR spectra of the synthesized compounds were recorded in DMSO using AV-300 BROKE JEOL Spectrophometer and tetramethylsilane (TMS) as an internal standard. The signals are expressed in δ ppm. The reactions progress was monitored by thin-layer chromatography (TLC) using silica gel G and spots were visualized in an iodine chamber.
Chemistry
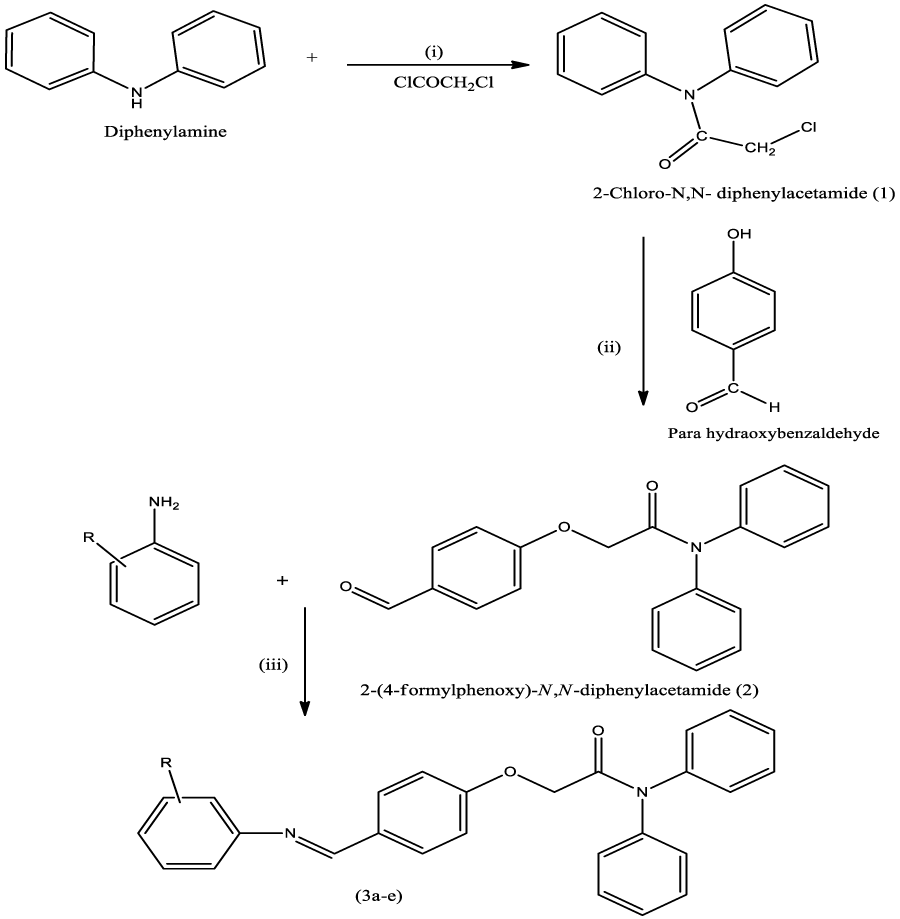
Synthesis of 2-Chloro-N, N-diphenylacetamide (1)
Diphenylamine (6.76gm, 0.004mol) was dissolved in toluene (200ml) in a 250ml round bottom flask and chloroacetylchloride (3.18ml, 0.004mol) was added. The reaction mixture was refluxed for 4hr. A total of 400ml of the water was then added to the reaction mixture and kept overnight for the precipitation of the product, which was filtered, washed with water, dried and recrystallized from ethanol to afford the colorless compound [12].
Synthesis of 2-(4-Formylphenoxy)-N, N-diphenylacetamide (2)
2-Chloro-N, N-diphenylacetamide (0.982gm, 0.004mole) was dissolved in acetonitrile (100ml) in a 250 ml round bottom flask and para hydroxy benzaldehyde (0.488gm, 0.004mol) was added. Anhydrous potassium carbonate (1.008gm 0.008mol) and catalytic amount of potassium iodide was added. The above reaction mixture was allowed to reflux with continuous stirring on magnetic stirrer for 16 hours. After completion of reaction, the reaction mixture was filtered and solvent was removed by vacuum distillation. The obtained product was recrystallized from ethanol and dried.
Yield: 65.45%; mp: 105oC; IR (cm-1): 3057 (C-H Str. Ar), 2919 (C-H Str. Ali), 1593(C=O Str.) 1315 (C-N Str.), 1250 (C-O-C Str.); 1H NMR (DMSO-d6): 4.66 (s ,2H,COCH2), 6.92-7.82 (14H, Ar), 9.87 (s,1H, CHO).
General Procedure for the Synthesis of various Schiff bases (3a-e)
2-(4-Formylphenoxy)-N, N diphenylacetamide (0.001mole) and appropriate aniline (0.001mol) was dissolved in an ethanol (100ml) in a 250ml of round bottom flask. The reaction mixture was allowed to reflux for 12h in the presence of glacial acetic acid (2-3 drops). The reaction mixture was cooled and kept overnight for precipitation. The precipitated product was filtered, recrystallized from ethanol and dried.
2-(4-(1-(Phenylimino) methyl) phenoxy)-N, N-diphenylacetamide (3a)
Yield: 74.50 %; mp: 180oC; IR (cm-1): 3019(C-H Str.Ar), 2920 (C-H Str.Ali), 1599(C=O Str.) 1403(C-N Str.), 1216(C-O-C Str.); 1H NMR (DMSO-d6): 4.68(s,2H,COCH2), 6.93-8.40 (19H,Ar), 9.87(s,1H,CHO).
Synthesis of 2-(4-(1-(4-Bromophenylimino) methyl) phenoxy) - N, N- diphenylacetamide (3b)
Yield: 77.40%; mp:210oC; IR (cm-1): 3019 (C-H Str.Ar), 2925(C-H Str.Ali), 1575(C=O Str.) 1321(C-N Str.),1249(C-O-C Str.); 1H NMR(DMSO-d6): 4.68 (s,2H,COCH2), 6.93-8.40 (18H,Ar), 9.90(s,1H,CHO).
Synthesis of 2-(4-(1-(2, 3-Dimethylphenylimino) methyl) phenoxy)-N, N-diphenylacetamide (3c)
Yield: 45.60%; mp: 160oC; IR (cm-1): 3056(C-H Str.Ar), 2921(C-H Str.Ali), 1606(C=O Str.) 1406(C-N Str.), 1253(C-O-C Str.); 1H NMR (DMSO-d6): 2.11(3H,CH3), 2.35(3H, CH3), 4.68(s,2H,COCH2), 6.93-8.29(17H, Ar), 9.90(s,1H,CHO).
Synthesis of 2-(4-(1-(4-chloro-2-nitrophenylimino) methyl) phenoxy)-N, N- diphenylacetamide (3d)
Yield: 75.70%; mp: 160oC; IR (cm-1): 3037(C-H Str.Ar), 2912(C-H Str.Ali), 1604(C=O Str.) 1305 (C-N Str.), 1251(C-O-C Str.); 1H NMR(DMSO-d6): 4.69(s,2H,COCH2), 6.13-8.14(17H,Ar), 9.90(s,1H,CHO).
Synthesis of 2-(4-(1-(4-chlorophenylimino) methyl) phenoxy)-N, N- diphenyl acetamide (3e)
Yield: 78.90%; mp: 185oC; IR(cm-1): 3037(C-H Str.Ar), 2920(C-H Str.Ali), 1579(C=O Str.) 1325(C-N Str.), 1251(C-O-C Str.); 1H NMR(DMSO-d6): 4.68(s,2H,COCH2), 6.61-8.36(18H,Ar), 9.90(s,1H,CHO).
Antibacterial activity
The antimicrobial activity of the compounds against human pathogenic Gram positive and Gram-negative bacteria was measured by measuring the zone of inhibition in disc diffusion method [13]. The synthesized compounds at 50 and 100µg/ml solutions in dimethylsulphoxide (DMSO) were evaluated in vitro for antibacterial activity against B. subtilis and E. coli [Table 1]. The measured zones of inhibition for the compounds ranged from 9-17 mm against B. subtilis and 8-19mm against E. coli. Based on zones of inhibition results, most of the compounds showed better activity against Gram negative bacteria than the activities against Gram negative bacteria. The potency has increased with group of 2-NO2 and 4-Cl at substituted aromatic ring (3d analogs) against Gram negative as compared to Gram positive bacteria. Ciprofloxacin was used as a standard antibacterial agent. Dimethylsulphoxide was used as a control and did not show any inhibition zones. Sterile Petri dishes of 90 mm diameter were used for antibacterial study.
Results and Discussions
Five Schiff bases were synthesized as outlined in Scheme 1 and evaluated for antibacterial activity. The results of antibacterial activity are shown in table 1. The title compounds (3a-e) were obtained by the reaction of diphenylamine with chloroacetylchloride in toluene afforded 2-chloro-N,N-diphenylacetamide (1) which react with 4-hydroxybenzaldyde in presence of potassium carbonate in acetonitrile afforded 2-(4-formylphenoxy)-N, N-diphenylacetamide (2), which on further reaction with various aniline in presence of glacial acetic acid in ethanol gave Schiff bases (3a-e). The structures of these compounds were established on the basis of M.P, TLC, IR and 1H-NMR spectral analysis. The title compounds were screened for their antibacterial activity and showed variable inhibition activities against the tested bacterial strains. Among the compounds tested, 2-(4-(1-(4-chloro-2-nitrophenylimino) methylphenoxy)-N, N-diphenyl acetamide (3d) showed maximum zone of inhibition 19mm against E.coli and 17mm against B. subtilis at 100µg/ml. The activity was due substitution of aromatic ring by 4-chloro and 2-nitro group that indicates evidence of selectivity of the ligand to the tested strain.
| Table 1: Antibacterial activity of target compounds (3a-e) against the microbes. | ||||
| Compounds | Zone of inhibition in mm | |||
| B. subtilis | E. coli | |||
| 50 µg | 100 µg | 50 µg | 100 µg | |
| Control | - | - | - | - |
| Ciprofloxacin | 22 | 25 | 22 | 25 |
| 3a | 10 | 13 | 11 | 16 |
| 3b | 12 | 11 | 14 | 15 |
| 3c | 16 | 14 | 13 | 16 |
| 3d | 15 | 17 | 17 | 19 |
| 3e | 09 | 12 | 08 | 11 |
Scheme 1: Synthesis of target compounds. Reagents and condition: (i) Toluene, Reflux 4hr (ii) Acetonitrile, Anhy. K2CO3, KI, Reflux (iii) Ethanol, Glacial acetic acid, Reflux.
References
- Cunha BA. Antibiotic resistancec. Drugs today. 1998; 34: 691-698. Ref.: https://goo.gl/CC8x73
- Lipsitch M. The rise and fall of antimicrobial resistance. Trends Microbiol.2001; 9: 438-444. Ref.: https://goo.gl/nvfwPt
- Sridhar SK, Muniyandy S, Ramesh A. Synthesis and antibacterial screening of hydrazones, Schiff and Mannich bases of isatin derivatives. Eur J Med Chem. 2001; 36: 615-625. Ref.: https://goo.gl/w4jLm1
- Kumar S, Kumar P, Sati N. Synthesis and biological evaluation of Schiff bases and azetidinones of 1-naphthol. J Pharm Bioall Sci. 2012; 4: 246-249. Ref.: https://goo.gl/KjV7gG
- Venugopal KN, Jayashree BS. Microwave-induced synthesis of schiff bases of aminothiazolyl bromocoumarins as antibacterials. Indian J Pharm Sci. 2008; 70: 88-91. Ref.: https://goo.gl/S2q1Nw
- Pandeya SN, Sriram D, Nath G, Declercq E. Euro. Synthesis, antibacterial, antifungal and anti-HIV activities of Schiff and Mannich bases derived from isatin derivatives and N-[4-(4'-chlorophenyl)thiazol-2-yl] thiosemicarbazide. J Pharm Sci. 1999; 9: 25-31. Ref.: https://goo.gl/vBgcuU
- Wadher SJ, Puranik MP, Karande NA, Yeole PG. Synthesis and Biological Evaluation of Schiff base of Dapsone and their derivative as Antimicrobial agents. Int J Pharm Tech Res. 2009; 1: 22-33. Ref.: https://goo.gl/Ut1AWU
- Bhat MA, Imran M, Khan SA, Siddiqui N. Biological Activities of Sulfonamides. J Pharm Sci. 2005; 67, 151-159. Ref.: https://goo.gl/6Miz3E
- Karthikeyan MS, Prasad DJ, Subrahmanya PB, Holla BS, Bioorg, et al. Synthesis and biological activity of Schiff and Mannich bases bearing 2,4-dichloro-5-fluorophenyl moiety. Bioorg Med Chem. 2006; 14: 7482-7489. Ref.: https://goo.gl/gTGQu2
- Li Y, Yang Z S, Zhang H, Cao B J, Wang F D, et al. Artemisinin derivatives bearing Mannich base group: synthesis and antimalarial activity. Bioorg Med Chem. 2003; 11: 4363-4368. Ref.: https://goo.gl/Cdihvu
- Wang L, Feng Y, Xue J, Li YJ. Ser Chem Soc. 2008; 73: 1-6.
- Kumar S, Singh R, Wahi A K. Synthesis, computational studies and preliminary pharmacological evaluation of 2-[4-(aryl substituted) piperazin-1-yl] N, N-diphenylacetamides as potential antipsychotics. Eur J Med Chem. 2011; 46: 4753-4759. Ref.: https://goo.gl/egioRh
- Bauer AW, Kirby WM, Sherries JC, Tuck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966; 45: 493-496. Ref.: https://goo.gl/42xj9v