Thesis
Retrosynthesis analysis; a way to design a retrosynthesis map for Pyridine and pyrimidine ring

Samar S Fatahala*
Pharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Helwan University, Egypt
*Address for Correspondence: Samar S Fatahala, Pharmaceutical Organic Chemistry Department, Faculty of Pharmacy, Helwan University, Ain-Helwan, Postal code: 11795, Helwan, Cairo, Egypt, Email: [email protected]
Dates: Submitted: 03 August 2017; Approved: 25 September 2017; Published: 26 September 2017
How to cite this article: Fatahala SS. Retrosynthesis analysis; a way to design a retrosynthesis map for Pyridine and pyrimidine ring. Ann Adv Chem. 2017; 1: 057-060. DOI: 10.29328/journal.aac.1001007
Copyright License: © 2017 Fatahala SS. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Abstract
Pyridine and pyrimidines are amongst the most important, well known heteroaromatic rings, owning to their bioactive importance. Herein, an idea about how to design the synthetic pathway for these rings using retrosynthesis analysis techniques.
Introduction
Chemical synthesis is exceptionally located at the heart of organic chemistry, or now a day used to call by medicinal chemistry; due to its influence on our lives and health [1]. For illustration, many of today’s medicines are synthetic, in predominantly are heterocyclic related compounds [2]. According to Corey 1989“ To the field of synthetic chemistry belongs an array of responsibilities which are crucial for the future of mankind, not only with regard to the health, material and economic needs of our society, but also for the attainment of an understanding of matter, chemical change and life at the highest level of which the human mind is capable”. The field of total (ideal) synthesis has a noticeable history and an inspiring future [3,4]. This report aimed to clarify the issue of how to design an ideal synthesis [5], for heterocyclic bioactive rings (namely; Pyrazole, Isoxazoles and imidazoles, as an example for 5-membered rings; Pyridine and Pyimidine, as an example for 6-membered). Compering between Retrosynthesis tree and ideal synthetic schemes.
Discussion
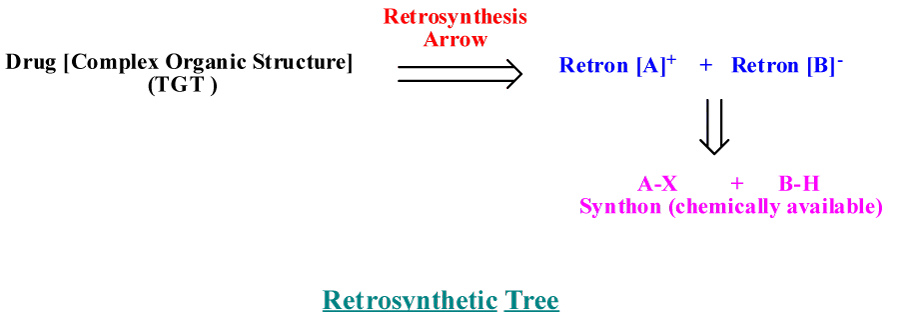
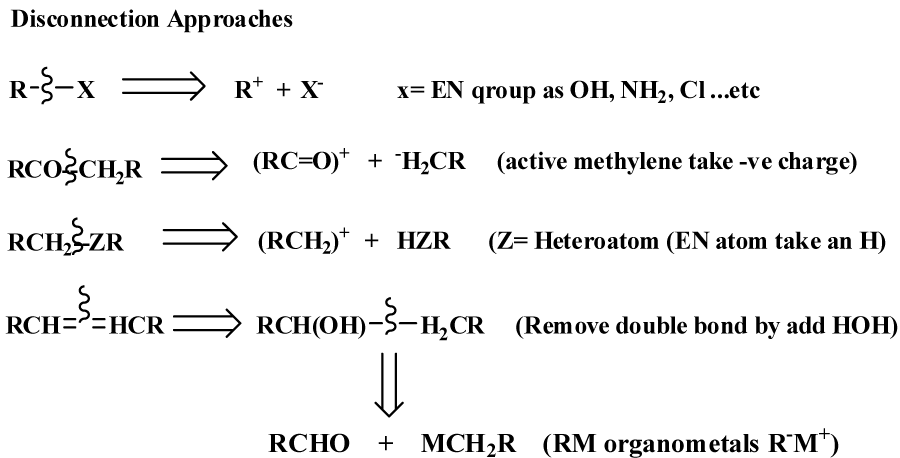
To prepare an organic compound (target, TGT), we need a readily obtainable starting materials and reagents. This process usually begins with the design of a synthetic plan (Strategy). Retrosynthetic analysis is a problem solving procedure for transforming the (TGT) gradually to more simpler structures (Synthon), through a pathway known as retrosynthesis tree, which finally leads to commercially available starting materials for a chemical synthesis [3], as revealed in figure 1. How to design a retrosynthesis tree First, Idea deal with where the disconnect occur [6-8], as revealed in figure 2. Second; for heterocyclic aromatic ring, we have to deal with saturated system, all unsaturated bonds must resolve. Third; synthetically equivalents synthons are to be given in its simplest way, synthetic equivalent in case of heteroaromatc ring mainly consist of active methylene and dicarbonyl compounds [9].
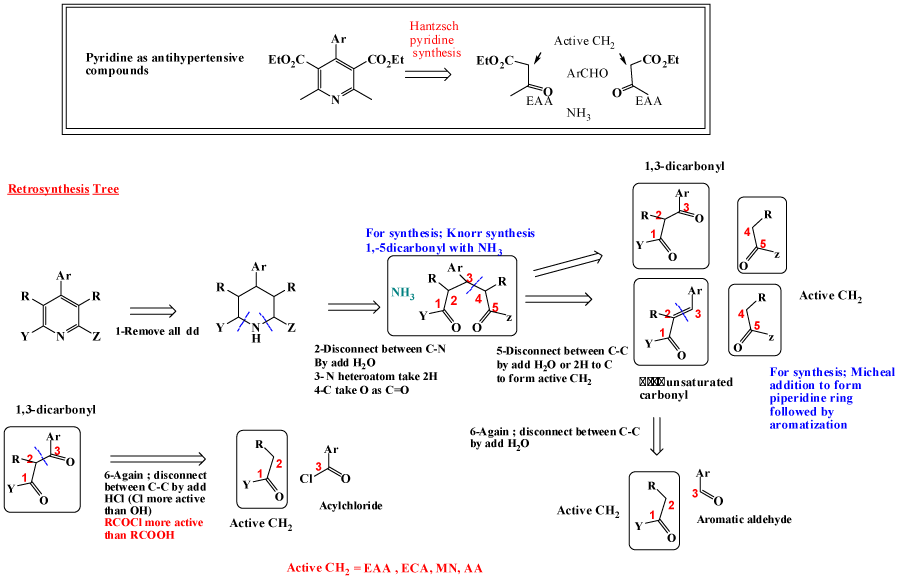
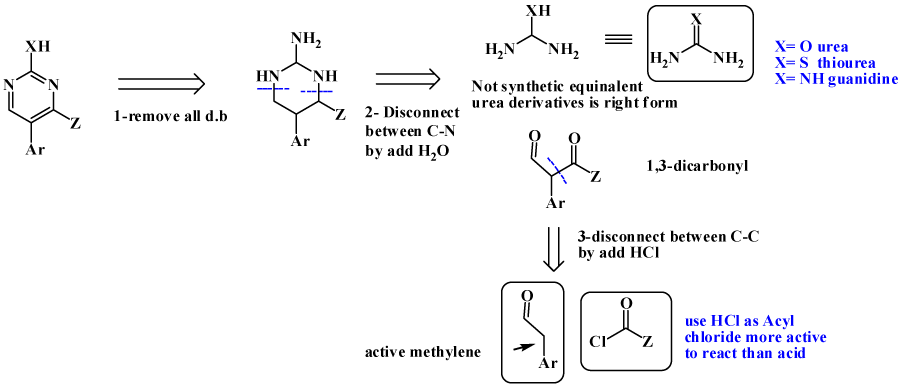
Pyridine as 6-membered heteroaromatic ring is one of most important bioactive compounds, naturally and synthetically occurred [10-12]. For Pyridine synthesis; main pathway is Hantzsch pyridine synthesis, where (α, β-unsaturated compound is added to active methylene as ethtyl acetoacetate (EAA) [13-17], also Knorr synthesis is available , by adding 1,5-dicarbonyl to heteroatom to give pyridine [18-20]. Micheal addition is also one of possible pathway [12,21]. Retrosynthesis tree of pyridine are discussed aside with its synthetically available pathways, is revealed in figure 3. Pyrimidine, is considered as most important ring as main components of DNA and privilege structure for dozen of bioactive drugs [22,23]. Using same steps as pyridine synthesis, as revealed in figure 4, retrosynthesis pathways for design pyrimidine synthesis. One of most applicable pathway is reaction between urea derivatives as (thiourea, guanidines) with 1,3-ducarbonyl compounds [24-28].
References
- Hoffmann RW. Elements of Synthesis Planning. Springer-Verlag, Berling Heidelberg. 2009.
- Corey EJ, Cheng XM. The Logic of Chemical Synthesis. John Wiley & Sons, NewYork. 1989.
- Gaich T, Baran PS. Aiming for the ideal synthesis. J Org Chem. 2010; 75: 4657-4673. Ref.: https://goo.gl/EkXnHX
- Wender PA. Toward the ideal synthesis and transformative therapies: the roles of step economy and function oriented synthesis. Tetrahedron. 2013; 69: 7529-7550. Ref.: https://goo.gl/sqdv1V
- Riera A. Asymmetric Synthesis of Nitrogen Heterocycles. Angew Chemie Int Ed. 2009; 48: 9590. Ref.: https://goo.gl/cfAKFj
- Burns MJ. Towards the Total synthesis of Phacelocarpus-2-pyrone A: Novel 2-pyrone chemistry. 2010. Ref.: https://goo.gl/cJPudn
- Godin F, Mochirian P, St-Pierre G, Guindon Y. Total synthesis of zincophorin methyl ester. Stereocontrol of 1,2-induction using sterically hindered enoxysilanes. Tetrahedron. 2015; 71: 709-726. Ref.: https://goo.gl/t8f1vE
- Pfefferkorn JA, Bowles DM, Kissel W, Boyles DC, Choi C, et al. Development of a practical synthesis of novel, pyrrole-based HMG-CoA reductase inhibitors. Tetrahedron. 2007; 63: 8124-8134. Ref.: https://goo.gl/i7p8sg
- Chen PS. Synthesis of Heterocyclic Natural Products and Analogues by Simon Fraser. 2012.
- Luo Y, Hu Y. Synthesis and antifungal activity of 2-aryl-1,2,4-triazolo[1,5-a]pyridine derivatives. Arch. Pharm. (Weinheim). 2006; 339: 262-266. Ref.: https://goo.gl/XB59Mc
- Mendes TCF, Raimundo JM, Nascimento-Junior NM, Fraga C, et al. Sedation and antinociception induced by a new pyrazolo[3,4-b]pyrrolo[3,4-d]pyridine derivative (LASSBio-873) is modulated by activation of muscarinic receptors. Pharmacol. Biochem. Behav. 2009; 94: 70-74. Ref.: https://goo.gl/W6sgqu
- Mohareb RM, Zaki MY, Abbas NS. Synthesis, anti-inflammatory and anti-ulcer evaluations of thiazole, thiophene, pyridine and pyran derivatives derived from androstenedione. Steroids. 2015; 98: 80-91. Ref.: https://goo.gl/UTGTPU
- Drug TN. Synthesis of heterocyclic compounds Heterocyclic compounds. Heterocycles. 2010.
- Fleita DH, Mohareb RM, Sakka OK. Antitumor and antileishmanial evaluation of novel heterocycles derived from quinazoline scaffold: a molecular modeling approach. Med Chem Res. 2012; 22: 2207-2221. Ref.: https://goo.gl/f4kmFT
- Hamada N, Abdo N. Synthesis, Characterization, Antimicrobial Screening and Free-Radical Scavenging Activity of Some Novel Substituted Pyrazoles. Molecules. 2015; 20: 10468-10486. Ref.: https://goo.gl/md94H2
- Miyamoto Y, Banno Y, Yamashita T, Fujimoto T, Oi S, et al. Design and synthesis of 3- pyridylacetamide derivatives as dipeptidyl peptidase IV (DPP-4) inhibitors targeting a bidentate interaction with Arg125. Bioorganic Med Chem. 2011; 19: 172-185. Ref.: https://goo.gl/mtdn2p
- Prakash O, Hussain K, Kumar R, Wadhwa D, Sharma C, et al. Synthesis and antimicrobial evaluation of new 1,4-dihydro-4-pyrazolylpyridines and 4-pyrazolylpyridines. Org Med Chem Lett. 2011; 1: 5. Ref.: https://goo.gl/mr7vM1
- Johnson DA, Keegan, David SSowell CG, Livesay MT, Johnson CL, et al. 3-O-Desacyl Monophosphoryl Lipid A Derivatives : Synthesis and Immunostimulant Activities † J Med Chem. 1999; 42: 4640-4649. Ref.: https://goo.gl/q4eNF4
- Mohamed MS, Fathallah SS. Pyrroles and Fused Pyrroles : Synthesis and Therapeutic Activities. Mini Rev Org Chem. 2014; 6: 477-507. Ref.: https://goo.gl/geVF6R
- Seela F, Shaikh KI. Oligonucleotides Containing 7-Deaza-2’-deoxyxanthosine: Synthesis, Base Protection, and Base-Pair Stability. Helv Chim Acta. 2006; 89: 2794-2814. Ref.: https://goo.gl/9J67fj
- Wardakhan WW, Samir EM. The reaction of cyclopentanone with cyanomethylene reagents: Novel synthesis of pyrazole, thiophene, and pyridazine derivatives. J Chem. 2013; 10. Ref.: https://goo.gl/CoUnYY
- Cordeu L, Cubedo E, Bandrés E, Rebollo A, Sáenz X, et al. Biological profile of new apoptotic agents based on 2,4-pyrido[2,3-d]pyrimidine derivatives. Bioorg Med Chem. 2007; 15: 1659-1669. Ref.: https://goo.gl/3T84te
- Qian X, Liu B, Wu Q, Lv D, Lin XF. Facile synthesis of novel mutual derivatives of nucleosides and pyrimidines by regioselectively chemo-enzymatic protocol. Bioorg. Med. Chem. 2008; 16: 5181-5188. Ref.: https://goo.gl/NeypS1
- Albuquerque H, Santos C, Cavaleiro J, Silva A. Chalcones as Versatile Synthons for the Synthesis of 5- and 6-membered Nitrogen Heterocycles. Curr Org Chem. 2014; 18: 2750-2775. Ref.: https://goo.gl/8Miacm
- Azam F, Alkskas I. a, Ahmed M. a. Synthesis of some urea and thiourea derivatives of 3-phenyl/ethyl-2-thioxo-2,3-dihydrothiazolo[4,5-d]pyrimidine and their antagonistic effects on haloperidol-induced catalepsy and oxidative stress in mice. Eur J Med Chem. 2009; 44: 3889-3897. Ref.: https://goo.gl/SMfVKi
- Holla BS, Mahalinga M, Karthikeyan MS, Akberali PM, Shetty NS. Synthesis of some novel pyrazolo[3,4-d]pyrimidine derivatives as potential antimicrobial agents. Bioorg Med Chem. 2006; 14: 2040-2047. Ref.: https://goo.gl/oehtnb
- Nofal ZM, Fahmy HH, Zarea ES, El-eraky W. Drug Synthesis Synthesis of New Pyrimidine Derivatives With Evaluation of Their Anti-Inflammatory and Analgesic Activities. Acta Po Pharm. 2011; 68: 507-517. Ref.: https://goo.gl/xTvHYF
- Shalaby AF, Abdulla MM, Amr AEGE, Hussain A. A Synthesis, Reactions, and Anti-arrhythmic activity of Substituted Heterocyclic Systems Using 5-Chloroanisic Acid as Starting Material. Monatshefte für Chemie-Chem Mon. 2007; 138: 1019-1027. Ref.: https://goo.gl/xgnG5B