More Information
Submitted: August 29, 2022 | Approved: September 12, 2022 | Published: September 13, 2022
How to cite this article: Gnanasekaran R, Petchiammal A, Subhashree BD, Anubha M, Dinakarkumar Y. Synthesis of citric acid using novel Aspergillus niveus obtained from agricultural wastes. Ann Adv Chem. 2022; 6: 051-055.
DOI: 10.29328/journal.aac.1001032
Copyright License: © 2022 Gnanasekaran R, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Aspergillus niveus; Citric acid; Extraction; Fermentation; Organic acid
Synthesis of citric acid using novel Aspergillus niveus obtained from agricultural wastes
R Gnanasekaran#, A Petchiammal, BD Subhashree, M Anubha and Yuvaraj Dinakarkumar*
Department of Biotechnology, Vel Tech High Tech Dr. Rangarajan Dr. Sakunthala Engineering College, Avadi, Chennai, Tamil Nadu, India
# Equal contributing author
*Address for Correspondence: Yuvaraj Dinakarkumar, Department of Biotechnology, Vel Tech High Tech Dr. Rangarajan Dr. Sakunthala Engineering College, Avadi, Chennai, Tamil Nadu, India, Email: [email protected]
Fungus belonging to the genus Aspergillus is considered highly important in the production of various types of enzymes and organic acids. Aspergillus species produce organic acids such as citric acid, itaconic acid, and malic acid, which are one of the most important alternate techniques for chemical processes. Citric acid is an important component in the manufacturing process of food and beverages, pharmaceuticals, cosmetics, toiletries, detergents, and other industries. In this work, A. niveus was isolated from the agricultural waste collected in Kotagiri, The Nilgiris, India. Submerged batch fermentation with a range of low-cost substrates, such as wheat flour, corn starch, and sweet potato, was used to successfully synthesize citric acid by the isolated fungus. In addition, production-related factors such as substrate concentration and incubation time were optimized. The maximum yield of citric acid was produced using A. niveus from corn starch at a concentration 7of 120 g/L after 168 hours at pH 3.2. Furthermore, with a degree of extraction of 91.96, citric acid was extracted from fermentation.
Aspergillus, filamentous fungi are essential for the production of bio-based organic acids. Due to their remarkable ability to secrete substantial volumes of desirable organic acids, a variety of Aspergillus strains belonging to phylogenetically distantly related species have been successfully employed in the industrial production of organic acids [1]. Several decades have been spent studying the mechanisms of organic acid biosynthesis in diverse Aspergillus species, as well as applying genetic engineering to improve the production of desirable organic acids [2-4].
Citric acid is one of the organic acids that can be used to make high-value products which include food and beverages, detergents, medicines, cosmetics, and toiletries, among other things. Because citric acid is a high-value product, it has recently gotten a lot of attention in terms of biological synthesis. Citric acid is utilized in a variety of industries, including food & beverage, detergents, medicines, cosmetics, and toiletries [5,6]. When Carl discovered this very valuable acid as a product in 1784, he was the first to describe it. Citric acid is used to manufacture food, synthetic resins, coatings, polymers, super-absorbents, phosphate-free detergents, and cleansers, among other things [7]. Petrochemical methods of producing citric acid have been phased out due to the depletion of fossil resources and the need for long-term development.
Citric acid has been fermented using a variety of fungus species, including Aspergillus niveus, Aspergillus niger, and others, with predictable effects. Aspergillus niveus species have been used to successfully generate citric acid [8]. A. niveus is important in the manufacture of a variety of valuable organic acids and enzymes in addition to citric acid. The Krebs cycle and the Embden-Meyerhoff-Parnos pathway are both used to produce citric acid, which is one of the intermediate products of the fungal metabolite TCA cycle [8]. Under some conditions, Aspergillus niveus, a genetically related species, can also synthesize citric acid [9].
In this research, a novel strain of A. niveus was identified for use in submerged batch fermentation to produce citric acid. Although A. niveus is known to produce enzymes, attempts have been made to manufacture organic acids from it. The fermentation broth was prepared using carbohydrate-rich source materials such as maize starch, corn starch, wheat flour, and sweet potato. To synthesize quality citric acid, the modification was carried out in the design of fermentation broth by infusing carbon-rich sources.
Microorganisms
The initial step of this work was to isolate a micro-organism from agricultural waste, which is capable of producing organic acid. For this work, agricultural waste samples were gathered near Kotagiri, The Nilgiris, India. A clean and dry sterile spatula was used to gather 10 g of sample for fungus isolation into clean polythene bags. Dirt suspension was made by dissolving 1 g of waste in 10 mL of sterile water. To isolate a single colony, serial dilution was used. Potato dextrose agar medium was used as a food source for the fungal species in this study. 28 g nourishing agar medium was dissolved in 1-liter deionized water and sterilized for 15 minutes at 121 °C in an autoclave. Single pure culture colonies were also obtained using the streak plate technique. 1 mL of 10-5 dilution from the sample was poured and spread over the Petri plates containing nutritional agar medium using a sterile “L-rod.” Mucous colonies began to grow across the culture plates after 4 - 7 days of culture at 36 °C (Orbitek, India). Finally, for identification reasons, the isolated fungal strain was sub-cultured.
Organic acid screening
The generation of organic acids was assessed by watching the color change of pH indicator plates as the fungi grew [3]. Microorganisms were shot up into the center of Petri dishes using Czapek-Dox agar media supplemented with 100 L per 100 mL triton X-100 as a surfactant and 1 g per 100 mL bromocresol green acts as a pH indicator. The pH of the medium was set around six. The plates were inoculated and incubated for 5 - 7 days at 30 °C, during which time a yellow color zone developed. The yellow zone’s area was later calculated, confirming the strain’s ability to generate organic acids.
Microorganism identification
18S rRNA sequencing with the universal primers 27F/1492R (ITS1 - 5’ TCCCGTAGGTGAACCTGCGG 3’ and ITS4 - 5’ TCCTCCGCTTATTGATATGC 3’) and 518F/800R (ITS1 - 5’ TCCCGTAGGTGAACCTGCGG 3’ and ITS4 - 5’ TCCTCCGCTTATTGATATGC 3’) and 518F/800R PCR amplifi-cation was used to copy the target DNA sequence. The PCR result will be utilized as a sequencing template. The sequence was amplified for 3 minutes at 96 °C (Initial denaturation). This procedure was carried out approximately 20 times at 96 °C for 40 seconds each time. The annealing and extension operations were also completed for 40 seconds at 70 °C and 2 minutes at 75 °C. The last extension took 15 minutes at 75 °C. Following PCR amplification, the DNA was sequenced using the traditional Sanger technique. The two strands of DNA (3’ to 5’ and 5’ to 3’) were sequenced using forward and reverse primers. The phylogenic tree was created with the help of a bioinformatics tool, and the sequence was compared to other sequences.
Batch fermentation and medium for seed culture
For the seed culture medium, the selected fungal species were used as seed, which in this case was potato dextrose agar (PDA), following the screening and selection of fungal strains. Citric acid was produced using fungal species that were cultured on plates. Corn starch, 140 g/L; MgSO4.7H2O, 2 g/L; NH4NO4, 3 g/L; nitric acid was used to alter the pH of the broth to 3.2. Citric acid was also produced using wheat flour, corn starch, and sweet potato.
Analytical methods
The cell mass concentration was calculated using the dry weight of the cells. By centrifuging the fermentation broth for 10 minutes at 10,000 x g and washing it thrice with deionized water, the cell mass in the broth was removed and dried at 75 °C until a uniform weight was achieved. The cells are subjected to centrifugation and later it was frozen up to -18 °C. To measure citric acid, we employed high-performance liquid chromatography (HPLC) with a column length of 0.3 m and a diameter of 7.8 mm operating at 39 °C with 0.005 M sulphuric acid as the mobile phase, a flow rate of 20 L min, and a UV–Vis diode array detector at 210 nm. All of the experiments were done in triplets in three different cultures.
Citric acid purification
One of the methods for isolating citric acid from fermentation broth is to extract it with an organic solvent (absolute ethanol). Acetone and several alcohols have been suggested as viable solvents. In certain solvents, citric acid dissolves quickly. However, the majority of contaminants in the fermentation broth, such as minerals, proteins, and sugars, are difficult to dissolve in those solvents. As a result, we used organic solvent extraction to purify citric acid as the first step in our citric acid purification method to eliminate the bulk of contaminants.
Screening of fungal strains for organic acids synthesis
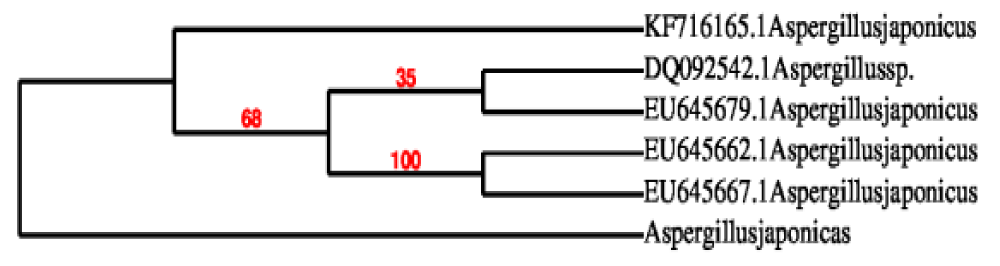
The ability to generate organic acids was tested in a total of 20 fungal strains. Six genera of fungus strains were identified which include Diaporthe, Fusarium, Aspergillus, Hypocrea, Penicillium, and Xylaria. The generation of organic acid in each of these 20 fungal strains was first determined using a plate test with a pH indicator. The establishment of an organic acid zone around the inoculation zone was noted, as was the formation of a yellow zone. Out of a total of 20 strains, the diameter of the yellow zone was measured for the six that had a favorable outcome. The most severe yellow zones were seen in four Aspergillus strains, followed by a Penicillium strain. As a result, these six strains were chosen to be examined further in submerged batch fermentation for organic acid production. Aspergillus species have been identified as useful for the industrial manufacturing of organic acids because of their higher production yield. Nonetheless, many Aspergillus species have been found to produce large amounts of citric and other organic acids. Several factors have led to the continued use of Aspergillus niger for commercial production, including its ability to grow and ferment a wide variety of cheap agro-industrial raw materials with higher yields, its powerful polymer degrading enzyme systems to hydrolyze many polymeric substrates [10]. After looking at six different fungal genera, we discovered that Aspergillus was the most promising for isolating organic acids. Furthermore, the isolated fungal strain was identified as Aspergillus niveus based on the 18 S rRNA sequence Figures 1,2 shows the growth of Aspergillus niveus in broth.
Figure 1: Phylogenetic analysis of Aspergillus niveus.
Figure 2: Aspergillus niveus Growth.
The effect of substrate concentration on the synthesis of citric acid
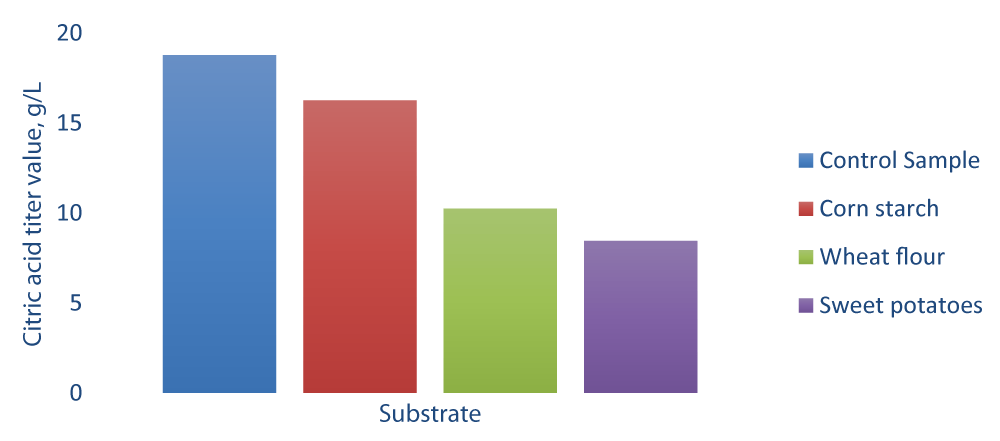
Aspergillus niveus produced more Citric acid in the corn starch medium than in the wheat flour or sweet potato medium. The necessary substrates for A. niveus growth and the production of the acid were provided. Samples were collected every 24 hours and citric acid was analyzed. The synthesis of citric acid was analyzed and controlled using pure glucose. The amount of citric acid produced by isolated A. niveus from diverse substrates such as corn starch, wheat flour, and sweet potato (Figure 3). A higher level of citric acid was produced by using corn starch as a substrate for the growth of A. niveus. Optimization of the substrate concentration was done using corn starch at various concentrations ranging from 50 g/L to 1000 g/L. The dry cell weight of fungi A. niveus was determined. It was discovered that substrate concentration has a considerable impact on the biomass’s dry cell weight. Table 1 shows the influence of different substrate concentrations on citric acid production. After 169 hours, the citric acid output from all three substrates (wheat flour, corn starch, and sweet potatoes) was 120 g/L, which is the maximal level of citric acid. A possible explanation is that the lower the number of starch granules, the lower the possibility of hydrogen ions penetrating amorphous regions. Especially in submerged fermentation processes, the composition of the medium strongly influences citric acid accumulation. Several factors influence citric fermentation, including carbon source type and concentration, nitrogen, and phosphate limitation, pH, aeration, trace element concentration, and microorganism morphology [11]. The citric acid quantity produced from corn starch, wheat flour, and sweet potatoes was found to be 15.25, 9.25 and 7.48 g/L, respectively.
Figure 3: Comparison of Citric acid production.
| Table 1: Effect of various substrate concentrations on citric acid production. | ||
| Substrate | Substrate concentration, g/L | Citric acid titer value, g/L |
| Corn starch | 50 | 14.24 |
| 100 | 16.25 | |
| 150 | 13.36 | |
| Wheat flour | 50 | 9.34 |
| 100 | 10.25 | |
| 150 | 9.22 | |
| Sweet potatoes | 50 | 7.02 |
| 100 | 8.45 | |
| 150 | 7.36 | |
Incubation period effect on acid production
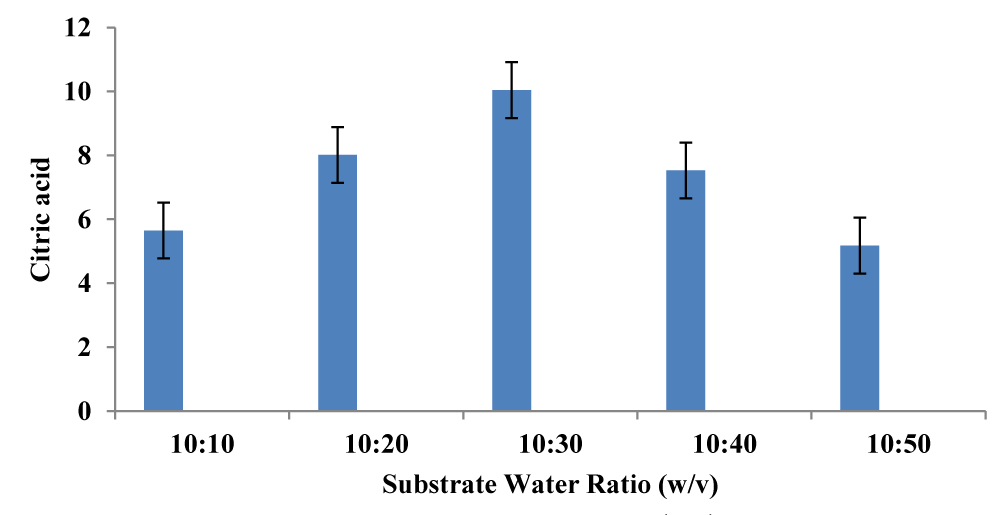
By varying the incubation time from 23 to 193 hours, Citric acid synthesis was investigated. With 15.65 g/L, maximum citric acid production was reported at 168 hours. Dallal, et al. (2021) achieved a similar production at 192 hrs [12]. The citric acid titer value initially increased with the increasing period (Figure 4). The incubation period is related to the generation of organic acid and other metabolic activities to some extent i.e., the amount of organic acid produced decreases with an increase in incubation time (Figure 5). More research is needed to learn about fed-batch fermentation for the production of citric acid utilizing A. niveus.
Figure 4: Effect of incubation period on citric acid production by A. niveus.
Figure 5: Optimization of the substrate to water ratio (w/v) ratio for citric acid using Aspergillus niveus.
Purification of citric acid
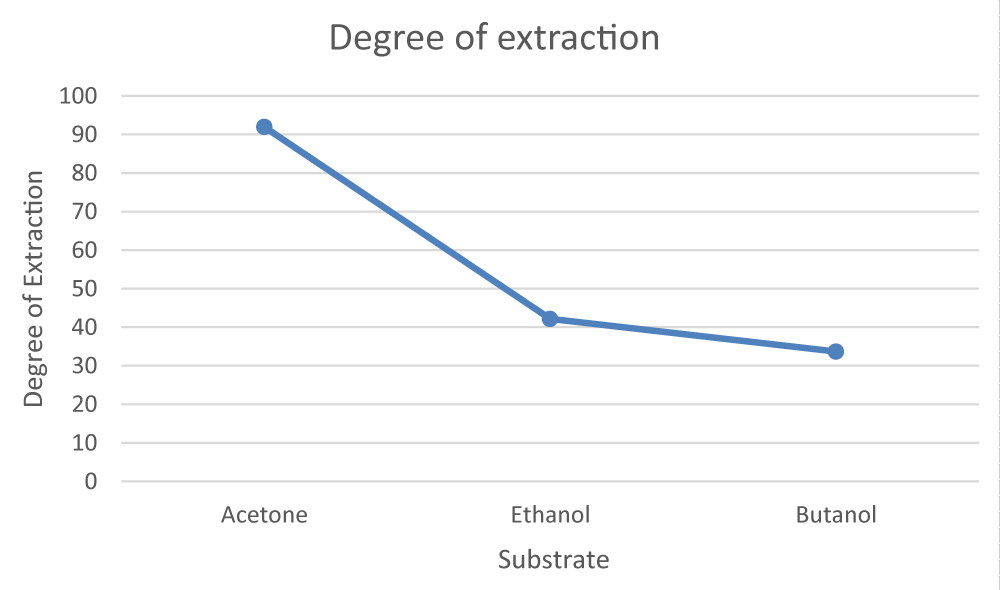
With the use of a separating funnel, Citric acid was purified from the broth using solvent extraction using acetone. The influence of the volume ratio between the organic and aqueous phases on obtaining maximal purification from the broth was investigated. The initial concentration of citric acid and the organic-to-aqueous volume ratio influence the degree of extraction of the acid. The acid-base titration was used in both the aqueous and organic phases are used to determine the concentration of purified citric acid. Table 2 shows that when a higher volume ratio of acetone to the beginning aqueous solution was utilized in the procedure, the degree of citric acid extraction increased dramatically [13-15]. The degree of extraction was maximum when the aqueous to organic ratio was 1:2, and afterward, it declined. As a result, Citric acid extraction with acetone should be done with a carefully chosen organic-to-aqueous volume ratio.
| Table 2: Degree of citric acid extraction. | |||||||
| Substrate | Volume (Initial, mL) | Volume (Equilibrium, mL) | Equilibrium Concentration (M) | Degree of Extraction | |||
| Aqueous | Solvent | Aqueous | Solvent | Aqueous | Solvent | ||
| Acetone | 10 | 15 | 10.6 | 15.5 | 5.25 | 26.68 | 74.38 |
| 10 | 25 | 9.4 | 25 | 3.5 | 29.52 | 85.54 | |
| 10 | 35 | 8 | 37 | 2.86 | 38.24 | 91.96 | |
| 10 | 45 | 4 | 48 | 5.2 | 29.15 | 89.65 | |
| Ethanol | 10 | 15 | 8 | 18 | 2.2 | 15.6 | 38.45 |
| 10 | 25 | 6 | 28 | 1.5 | 17.8 | 40.21 | |
| 10 | 35 | 2 | 37 | 0.9 | 23.76 | 42.15 | |
| 10 | 45 | 1 | 49 | 1.2 | 24.10 | 40.98 | |
| Butanol | 10 | 15 | 7 | 16 | 0.9 | 12.07 | 31.57 |
| 10 | 25 | 4 | 28 | 0.6 | 15.5 | 33.01 | |
| 10 | 35 | 4 | 38 | 0 | 19.54 | 33.69 | |
| 10 | 45 | 2 | 49 | 0.36 | 17.82 | 33.24 | |
An efficient and low-cost medium for the synthesis of citric acid was developed using isolated A. niveus fungal strain obtained from agricultural wastes. Initial screening procedures revealed that Aspergillus species can be used to promote effective Citric acid synthesis. According to several testing methods, the submerged fermentation of maize starch at a concentration of 120 g/L revealed A. niveus to be one of the producers of citric acid. Since citric acid is an extremely valuable product that is in demand, in both the food and pharmaceutical industry worldwide, agricultural wastes can gain another shelf life. In addition, in order to reduce the cost of production of the citric acid by the Aspergillus niger, agricultural wastes generated by many countries can be used as a reliable source of substrate material for its production. Thus the novel A. niveus fungal strain was used in the synthesis of citric acid. An average quantity and quality of synthesis were achieved from the above method.
- Yang L, Lübeck M, Lübeck PS. Aspergillus as a versatile cell factory for organic acid production. Fungal Biology Reviews. 2017; 31(1):33-49.
- Bennett JW. An overview of the genus Aspergillus. Aspergillus: molecular biology and genomics. 2010; 1-7.
- Sun X, Wu H, Zhao G, Li Z, Wu X, Liu H, Zheng Z. Morphological regulation of Aspergillus niger to improve citric acid production by chsC gene silencing. Bioprocess Biosyst Eng. 2018 Jul;41(7):1029-1038. doi: 10.1007/s00449-018-1932-1. Epub 2018 Apr 2. PMID: 29610994.
- Alekseev VK, Dubina MV, Komov VP. Molecular-genetic and biochemical characteristics of citrate synthase from the citric-acid producing fungus Aspergillus niger. Applied Biochemistry and Microbiology. 2016; 52(9):810-817.
- Kubicek CP, Röhr M, Rehm HJ. Citric acid fermentation. Critical Reviews in Biotechnology. 1985; 3(4):331-73.
- Li G, He D, Qian Y, Guan B, Gao S, Cui Y, Yokoyama K, Wang L. Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int J Mol Sci. 2012;13(1):466-76. doi: 10.3390/ijms13010466. Epub 2011 Dec 29. PMID: 22312264; PMCID: PMC3269698.
- Kumar D, Jain VK, Shanker G, Srivastava A. Citric acid production by solid-state fermentation using sugarcane bagasse. Process Biochemistry. 2003; 38(12):1731-1738.
- Vandenberghe LP, Soccol CR, Pandey A, Lebeault JM. Solid-state fermentation for the synthesis of citric acid by Aspergillus niger. Bioresource Technology. 2000; 74(2):175-8.
- Shishikura A, Takahashi H, Hirohama S, Arai K. Citric acid purification process using compressed carbon dioxides. The Journal of Supercritical Fluids. 1992; 5(4):303-312.
- Papagianni M. Advances in citric acid fermentation by Aspergillus niger: biochemical aspects, membrane transport and modeling. Biotechnol Adv. 2007 May-Jun;25(3):244-63. doi: 10.1016/j.biotechadv.2007.01.002. Epub 2007 Jan 19. PMID: 17337335.
- Max B, Salgado JM, Rodríguez N, Cortés S, Converti A, Domínguez JM. Biotechnological production of citric acid. Braz J Microbiol. 2010 Oct;41(4):862-75. doi: 10.1590/S1517-83822010000400005. Epub 2010 Dec 1. PMID: 24031566; PMCID: PMC3769771.
- Chergui D, Akretche-Kelfat S, Lamoudi L, Al-Rshaidat M, Boudjelal F, Ait-Amar H. Optimization of citric acid production by Aspergillus niger using two downgraded Algerian date varieties. Saudi J Biol Sci. 2021 Dec;28(12):7134-7141. doi: 10.1016/j.sjbs.2021.08.013. Epub 2021 Aug 9. PMID: 34867016; PMCID: PMC8626340.
- Kulprathipanja S. Inventor; UOP LLC, assignee. Separation of citric acid from fermentation broth with a neutral polymeric adsorbent. United States patent US 4,720,579. 1988.
- Wu J, Peng Q, Arlt W, Minceva M. Model-based design of a pilot-scale simulated moving bed for purification of citric acid from fermentation broth. J Chromatogr A. 2009 Dec 11;1216(50):8793-805. doi: 10.1016/j.chroma.2009.03.028. Epub 2009 Mar 17. PMID: 19344909.
- Zhu S, Zhao X, Song Y, Lu S, Yang B. Beyond bottom-up carbon nanodots: Citric-acid derived organic molecules. Nano Today. 2016. 11(2):128-132.