More Information
Submitted: October 04, 2023 | Approved: October 16, 2023 | Published: October 17, 2023
How to cite this article: Peña DCM, Anguiano ACR, Casillas MC, Ramírez APV, Fonseca MMF.et al. Qualitative Identification of Secondary Metabolites and Determination of the Toxicity of Extracts Obtained from the Flower of Kalanchoe Pinnata. Ann Adv Chem. 2023; 7: 068-073.
DOI: 10.29328/journal.aac.1001046
Copyright License: © 2023 Peña DCM, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Keywords: Kalanchoe pinnata; Flower; Extracts; Secondary metabolites; Toxicity
Qualitative Identification of Secondary Metabolites and Determination of the Toxicity of Extracts Obtained from the Flower of Kalanchoe Pinnata
Diana Carina Martínez Peña1, Ana Cristina Ramírez Anguiano1, Martha Cueto Casillas1, Ana Paulina Velasco Ramírez2, Milagros Melissa Flores Fonseca3, María Adriana Delgado Armas1, Ana Lucía Camacho Larroca1 and Sandra Fabiola Velasco Ramírez1*
1Department of Chemistry, University Center of Exact Sciences and Engineering, Blvd. Marcelino García Barragán #1421, Guadalajara, Jalisco, 44430, Mexico
2Department of Agricultural Production, University Center for Biological and Agricultural Sciences (CUCBA), University of Guadalajara, Zapopan 45010, Mexico
3Doctorate in Medical Science, University of Colima, Avenida Universidad 333, Las Víboras, 28040, Colima, Colima, Mexico
*Address for Correspondence: Sandra Fabiola Velasco Ramírez, Department of Chemistry, University Center of Exact Sciences and Engineering, Blvd. Marcelino García Barragán #1421, Guadalajara, Jalisco, 44430, Mexico, Email: [email protected]
Kalanchoe pinnata, also known as air leaf or life leaf, is a plant used in traditional medicine in different world regions. In Mexico, it is included in the Atlas de la Medicina Tradicional Mexicana with a wide variety of applications, such as antimicrobial, anti-inflammatory, anticancer, and antihistamine, among others. However, neither a secondary metabolite profile of the flower has been reported nor information on its possible toxicity. The latter is the purpose of this work. A phytochemical profile of extracts with solvents of different polarity (aqueous, methanol, ethanol, and ether) was carried out. In this profile, the structural compounds could be qualitatively determined by chemical reactions, and some changes in coloring or precipitation were observed. The acute toxicity test of the extracts was performed with an aquatic organism, Artemia sp, and a terrestrial organism Eisenia foetida, as well as the evaluation of the antioxidant capacity of the extracts in the organism of Eisenia foetida. The ABTS radical method and TROLOX were applied as synthetic antioxidants for the evaluation of the inhibition percentage. Most important secondary metabolites were qualitatively identified in the extracts of K. pinnata flowers. Mainly in the alcoholic extracts (methanol and ethanol) tannins, alkaloids, and flavonols were found. As mentioned above, they are reported to have toxicological effects. The toxicity and antioxidant activity tests confirm the preliminary results obtained in the identification of secondary metabolites. It is therefore concluded that the flower of Kalanchoe pinnata contains secondary metabolites that may be of great therapeutic interest.
In Mexico, plants are a source of alternative medicine because their secondary metabolites are associated with healing properties for a number of diseases, among these properties we mention: anticancer, anti-inflammatory, antihistamine, antimicrobial, and so on [1]. Due to this large field of application, interest has grown in analyzing the chemical constituents that might be associated with biological activities, such as antioxidant activity and toxicity.
In the case of Kalanchoe pinnata leaves, studies have reported the presence of bufadienolides, which have been linked to cytotoxic, antitumor, and cardiotonic activities [2]. Furthermore, it has been mentioned that tannins are also present, which contribute to anthelmintic activity [3], and antimicrobial activities. However, there is no scientific evidence on the composition and biological activities of the flowers of this species.
Phytochemical screening is one of the initial steps in the chemical investigation of organic compounds, allowing qualitative determination of the main groups of chemical constituents present in a plant and subsequent extraction or fractionation of the extracts for isolation of the groups of interest [4].
Screening involves the extraction of the plant using appropriate solvents and the application of staining or precipitation reactions. These types of chemical tests are very useful as they are characterized by their specificity, rapidity, and cost-effectiveness. Photochemical analysis detects the main types of metabolites related to some biological activity, such as alkaloids, flavonols, carbonyl compounds, sugars, and tannins, among others [1].
It is well known that plants can produce metabolites that cause toxic reactions in those who consume them. This is why research is carried out with the aim of determining the toxicity of medicinal plants.
By definition, acute toxicity refers to adverse effects following intake of a single oral or dermal dose of a substance of concern, administered over 24 hours. The LC50 is defined as the concentration of a toxic material lethal to 50% of the test organisms. Artemia salina larvae have been used in bioassays by numerous laboratories in different parts of the world, and the results of these analyses in the determination of LC50 are reliable and replicable [6]. This method is an initial step in determining the toxicity of a substance of interest. When a positive result was obtained, the contact toxicity test with earthworm Eisenia foetida was followed [5]. This method involves the contact of the earthworms with filter paper exposed to the substances to be analyzed for 24 and 48 hours, in order to identify the toxic chemical potential and the side effects that the organism may be exposed to when in contact with the extracts of interest [7].
Sample collection
The plant material was collected in the months of June 2018 and February 2019 manually, picking the flowers of the plant by cutting them with the aid of conventional scissors. The plant material obtained was taken to a freeze-drying process in a LABCONCO freeze-dryer at a temperature of -45° and pressure of 0.040 μbar for 24 hours and stored in hermetically sealed bags in a place free from moisture and light.
btaining the extracts
Subsequently, 1.5 grams of the dry material was weighed in an analytical balance, then crushed in a mortar until the homogeneous size and 50 mL of solvent (water, methanol, ethanol, and ether) and added and left to macerate for 72 hours, stored at 4 °C in a sealed container wrapped in aluminum foil to protect it from light.
After this time, the extracts were filtered by gravity, the liquid phase was recovered and stored at 4 °C and protected from light.
Phytochemical marking
For the rapid identification of unknown organic compounds in the sample, several chemical reactions can be performed to examine their functional groups. These tests give a broad overview of the possible structures present in the extracts, by observing color changes, precipitates, or vapor release, which are characteristic of each reaction. A total of 10 secondary metabolites were determined, including phenols, flavonoids, tannins, alkaloids, sugars, proteins, amino acids, cholesterol, saponins, and coumarins, following the suggested methods of Tiwari [1]. Each test was performed and controlled in triplicate in individual test tubes.
Phenols: For determination with the ferric chloride test, 0.5 mL of the extract and 0.5 mL of 5% FeCl was added to a test tube. The presence of a red, blue, green, or purple color indicates the presence of phenols. Another test for the identification of phenols in an organic sample is the alkali addition which consists of the addition of 5 drops of 20% NaOH to 0.5 mL of the extract, the formation of purple, blue, or green color indicates the presence of alkaline phenols and a yellow coloring indicates the presence of betacyanins [1].
Flavonoids: The Salkowski test consists of adding 0.5 mL of extract and 0.25 mL of chloroform with a subsequent shaking, followed by adding 1 drop of concentrated HCl. The result is positive for flavonoids if yellow coloring is observed; for flavones with orange-green coloring, for chalcones with red to blue coloring, and the presence of quinones with red-purple coloring.
The Shinoda test was also performed where a piece of magnesium chip and five drops of concentrated HCl were added to a tube containing 0.5 mL of the extract. The appearance of colors ranging from red to magenta indicates the presence of a flavonone or dihydroflavonol.
Tannins: The gelatin test was performed by adding 0.5 mL of extract, and 5- 10 drops of gelatine solution (2.5 g of pure grenetin hydrated in NaCl solution and standardised in 100 mL of water). For the detection of tannins, the appearance of a precipitate [1] is considered positive.
Alkaloids: Identification was performed by using different reagents such as Dragendroffs reagent, Mayers, Wagner, and Hager’s reagent.
Dragendroffs: 5 to 10 drops of 10% HCl and 3 drops of Dragendroffs reagent are added to 0.5 mL of the extract. The test is positive for alkaloids when a red, orange, or brown precipitate persists for 24 hours.
Mayers: To 0.5 mL of the extract add 5 to 10 drops of 10% HCl, shake, and add 0.25 mL of Mayers solution. Most alkaloids react by providing a white or light yellow, amorphous, or crystalline precipitate.
Wagner: To 0.25 mL extract add 5 to 10 drops of 10% HCl, shake, and add 0.25 mL Wagner reagent. The test is positive for alkaloids if a brown precipitate is observed.
Hagers: To 0.25 mL of the extract, 5 to 10 drops of 10% HCl are added and 1.25 mL of Hagers solution is added. The formation of a precipitate indicates the presence of alkaloids [1].
Sugars: Different tests were performed with the reagents: Benedict. Fehling, Keller-Killiani and Molisch.
Benedict: To 0.25 mL of extract, 0.25 mL of Benedict’s reagent is added, heated in a water bath, and allowed to cool. It identifies reducing sugars in alkaline solutions, by means of the precipitation of red-orange color.
Fehling: To 0.25 mL of each extract add 0.5 mL of Fehling’s solution A and 0.5 mL of Fehling’s solution B, and heat in a water bath for a few minutes.
It is based on the reducing properties of the carbonyl group of the aldehydes. This oxidizes to acid and reduces the copper salt in an alkaline medium to copper oxide, forming a red precipitate.
Keller-killiani: 0.25 mL of the extract is mixed with 0.25 mL of glacial acetic acid and a drop of 2% FeCl2, then 0.25 mL of concentrated H2SO4 is added. The presence of a reddish-brown ring at the interface identifies the presence of deoxy sugars [1].
Proteins: The Xanthoprotein test was performed, which identifies proteins with aromatic groups that are derived from benzene through the formation of yellow-nitrated compounds. To 0.25 mL of the extract add 10 drops of concentrated HNO3, place in a water bath for 5 minutes, and allow to cool [1].
Amino acids: Tests were carried out with lead acetate and ninhydrin.
Lead acetate: to 0.5 mL of extract add 0.3 mL of distilled water and 0.5 L of 5% lead acetate. Sulfur-containing amino acids can be recognized by the formation of dark grey or black lead sulfide precipitates1.
Ninhydrin: Take 1.5 mL of the extract and add 0.25 mL of 0.1% nihnhydrin solution. Mix, heat to boiling in the flame of the burner, and allow to cool. The alpha-amino group of amino acids will form colored complexes with ninhydrin; bluish violet for most amino acids whose amino group is primary, yellow for proline and hydroxyproline, and brown for asparagine which has an amido group in the side chain1.
Cholesterol: Libermann Buchard’s test was performed. To 0.25 mL of the extract, 0.25 mL of acetic anhydride is added and shaken. Then 2 to 3 drops of concentrated H2SO4 are added. The colors start at a purplish pink and progress through light green and then to deep Green [1].
Saponins: Foam test on the sample. Place 0.5 mL of extract in a tube and 2 mL of distilled water, shake manually, and vigorously for 30 seconds. The formation of honeycomb-shaped foam is considered positive for saponins.
Coumarins: Make a 9:1 extract/water dilution. Take 2 mL and place it in a test tube with a stopper. Take a strip of filter paper dipped in NaOH (0.06 g/mL) and place it in the tube. Heat in a Bunsen burner until vapors are released. Remove the filter paper and observe under UV light. Then, if fluorescent substances are present, the test is positive for coumarins.
Acute toxicity bioassay with Artemia salina
Artemia spp. Are tiny, soft-bodied, caramel-colored, light-transparent shrimps belonging to the Phylum Arthropoda, class Crustaceae, subclass Branchiopoda. They are commonly known by the name artemia, also called “sea monkeys” or “brine shrimp “ [8]. Artemia spp. larvae have been used for more than 40 years in toxicological and ecotoxicological studies [9,10] and their biology and potential uses in various fields have been studied as a practical and economical method for the determination of bioactivity of synthetic compounds and natural products [11]. For this essay groups of 15 nauplii of Artemia salina were collected using a Pasteur pipette and added to different wells of a microplate. They were exposed to concentrations of 10, 20, and 35 mg/mL of K. pinnata extracts for 24 h at room temperature with constant agitation and under a continuous light regime. Each test was performed in triplicate. At the end of 24 h of exposure, the number of dead organisms was counted and the percentage mortality was calculated. Larvae are considered dead if they do not exhibit movement during several seconds of microscopic observation.
The rank of toxicity of the extracts is determined in terms of the range in which the LC50 values were found according to the following categories: extremely toxic (LC50<10 μg/mL), very toxic (10< LC50<100), moderately toxic (100< LC50<1000) and non-toxic (LC50>1000μg/mL) [12].
Acute toxicity bioassay on earthworm Eisenia foetida
For the toxicity test, adult earthworms of the species Eisenia foetida with clitella (obtained from Lombricompost, Guadalajara, Jalisco, México) were used, according to the recommendations by the Organization for Economic Cooperation and Development (OECD) [7]. All adult earthworms had a well-defined clitellum and weighed from 250 to 400 mg. They were kept under dark conditions and at a controlled temperature of 21 °C ± 1 °C in culture chambers, maintaining extreme care in handling during all experiments.
The acute toxicity test was performed according to OECD guidelines [7]. Extracts of K. pinnata flower with different solvents at various concentrations (1, 5, and 10 mg/mL) were used for the acute toxicity test. After placing individual pieces of filter paper on the bottom of the Petri dishes, 1 mL of each extract of known concentration was added, one with deionized water, and one more of the solvent of each extract as controls to wet the entire filter paper. Then, one earthworm was randomly placed in each Petri dish. Four replicates will be used for each treatment. Petri dishes were incubated in the dark at 25 °C ± 1 °C for 48 h and mortality was recorded every 24 h; worm weight and morphological changes were also observed. Mortality (%) in the worm will be evaluated to determine the LC50.
Euthanasia
At the end of the test, the surviving animals were sacrificed in an atmosphere of 20 dm3 min-1 of CO2 for 15 min.
Evaluation of antioxidant capacity
All worms participating in the toxicity test after the euthanasia are collected immediately after the experimentation. They are placed in falcon tubes with 5 ml of phosphate buffer solution (PBS) taking strict care not to contaminate the sample, avoid contact with light, and keep refrigerated for no more than one week. Using a tissue macerator, the replicates of each experiment (3 worms per experiment/extract) were ground to obtain a second homogeneous extract, then, this extract was centrifuged for 10 minutes at 3500 rpm. The supernatant was collected in stoppered tubes protected from light and refrigerated at 4°C until further analysis. Preparation of the ABTS radical (2,2, azinobis-3-ethyl-benzothiazolin-6-sulphonic acid): 96 mg ABTS and 12.8 mg ammonium, sodium or potassium persulphate are weighed and gauged to 25 ml with distilled water. Allow the radical to activate for 16 hours under refrigeration and protect it from light. After activation, the radical is diluted with water to an absorbance of 0.7 ± 0.02 at 750 nm. A calibration curve was prepared with the synthetic antioxidant TROLOX (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) using the ABTS radical with an absorbance of 0.7 ± 0.02 at 750 nm. The range of the curve is from 0 to 400 mM Trolox. In a 96-well plate, 20 mL of each point of the curve in triplicate, plus 280 mL of the previously prepared and standardized ABTS radical, are placed in a 96-well plate. Subsequently, it is placed in the spectrophotometer and its absorbance is measured at 750 nm. The samples were read in the spectrophotometer in the same way, placing 20 mL of each point of the curve in triplicate, plus 280 mL of the ABTS radical, and reading at 750 nm every 5 minutes until the absorbance reading was stable for all samples.
Extracts of the freeze-dried flower of K. pinnata were obtained in aqueous medium, methanol, ethanol, and ether. Phytochemical profiling was carried out from these extracts for the qualitative identification of the secondary metabolites present in the flower. The results obtained are shown in Table 1.
| Table 1: Results of the qualitative profile for organic compounds in the extracts of K. pinnata in wáter. | |||||
| Test | Water | Methanol | Ethanol | Ether | |
| Phenols | Ferric chloride | (-) | (+) | (-) | (-) |
| Álcalis | (+) | (+) | (++) | (-) | |
| Flavonoids | Salkowski | (+) | (+) | (+) | (-) |
| Shinoda | (+) | (++) | (++) | (-) | |
| HCl addition | (+) | (+) | (++) | (-) | |
| Tannins | Gelatin | (++) | (++) | (++) | (-) |
| Alkaloids | Dragendroffs | (-) | (-) | (++) | (-) |
| Mayers | (-) | (-) | (++) | (-) | |
| Wagner | (-) | (-) | (+) | (+) | |
| Hagers | (-) | (-) | (++) | (-) | |
| Carbohydrates | Molisch | (+) | (++) | (+) | (+) |
| Benedict | (+) | (+) | (+) | (-) | |
| Fehling | (-) | (++) | (+) | (-) | |
| Keller-K | (-) | (+) | (++) | (-) | |
| Proteins | Xanthoprotein | (++) | (++) | (++) | (+) |
| Amino acids | Pb acetate | (+) | (+) | (+) | (-) |
| Ninhydrin | (++) | (++) | (+++) | (-) | |
| Cholesterol | Liberman B. | (-) | (-) | (-) | (-) |
| Saponins | Foam | (-) | (-) | (-) | (-) |
| Quinones | H₂SO₄ | (+) | (+) | (+) | (-) |
| Methanol, ethanol and ether (+++ Very abundant, ++ Abundant, + not very abundant, - Not detected). | |||||
As can be seen in Table 1, the ethanolic extract was the one showing the best results, as a higher presence of secondary metabolites was observed, of which phenols, flavonols, tannins, alkaloids, amino acids, and proteins stand out. This was followed by the aqueous extract and finally the ether extract. The ethanolic extract was followed by the methanol and aqueous extracts, which displayed the presence of the same metabolites, but to a lesser extent. Finally, only sugars, alkaloids, and proteins were found in the ether extract. Within the colorimetric tests, it was observed that the phenolic composition (phenols, flavonoids, and tannins) predominates more than the rest of the metabolites studied. Many properties have been attributed to these compounds, including antioxidants [13], antimalarial [14], anti-inflammatory [15,16], antimicrobials [14], and antifungals [17], among others. With these attributes, the applications of the extract could be diverse, especially in the pharmaceutical and food industries. Additionally, its application could be easier due to the absence of saponins and alkaloids, in aqueous extracts since it is speculated that these metabolites are one of the main toxic ones for humans [18]. However, this does not apply to the ethanolic extract where the presence of alkaloids is observed abundantly. Let us remember that alkaloids have very diverse chemical structures; they generally act on the central nervous system, such as morphine in Papaver somniferum, which is an anesthetic, or caffeine in Coffea arabica and nicotine in tobacco, Nicotiana tabacum, which are stimulants of the System Central Nerve. Other alkaloids have activity on the autonomic nervous system, such as pilocarpine from jaborandi (Pilocarpus sp., Rutaceae) leaves, with parasympatholytic properties, atropine from Atropa Belladonna, with anticholinergic activity, or scopolamine from Hyosciamus albus, which acts as a competitive antagonist of the substances. That stimulate the parasympathetic. Therefore, when making herbal preparations such as tinctures that, as a method of extracting the active ingredients of a plant, are made by leaving the plant in ethyl alcohol or ethanol, care must be taken [19].
It was not possible to determine the LC50 in alcoholic extracts (methanol* and ethanol, N/A = Not Available) in the Eisenia foetida assay because the concentrations of the samples were high and the organisms in the experiment did not survive even for 24 hours to carry out a pertinent observation (Table 2). Thus, it was not feasible to calculate this parameter. Meanwhile, the methanolic extract in the Artemia sp assay was not yet considered at the time of the experiment. And finally, with the outbreak of the COVID-19 pandemic, the laboratory on-site activities were canceled, making it impossible to complete the assay.
Table 2 shows the relationship between the results of the qualitative profile and those of the LC50. It is understood that the alcoholic extracts, containing the highest amount of secondary metabolites, are the ones that had the most detrimental effect on the Eisenia foetida reptids subjected to the test, so they died before the observation time proposed. The alcoholic extracts were followed by the ethereal extract with an LC50 of 17706 and 2159.59 ppm respectively for Artemia and Eisenia foetida, however, as they did not show a significant number of positive results in the qualitative analysis, it can be concluded that the toxicity is mainly due to the solvent, as the control had very similar behavior to the organisms tested. Finally, the aqueous extract showed lower toxicity, resulting in LC50 of 118412 and 3149 ppm respectively for Artemia and Eisenia foetida. This result may be related to the presence of polyphenolic compounds, a group to which tannins belong. In this regard, it has been reported that hydrolyzable tannins are potentially toxic and can affect the liver and kidneys, possibly causing death [20]. Hydrolyzable tannins are said to be responsible for most of the harmful effects because they can be absorbed and circulated through the bloodstream [21,22]. On the other hand, animals that consume plants with high levels of condensed tannins decrease nutrient utilization, affecting protein use to a large extent. The biological activity of condensed tannins depends on two main factors: their concentration and structure [23]. In a study conducted by Bogucka-Kocka, et al. where of all the Kalanchoe species that they studied, they reported that the extract obtained from K. pinnata leaves showed high cytotoxicity against the H-9 cell line and T cells, this possibly attributed to polyphenolic compounds such as flavonoids and phenolic acids. Another secondary metabolite that can cause toxicity may be the alkaloids in the extract since toxic properties have been attributed to these compounds. However, this analysis only gives an estimate of the mean lethal concentration; more analyses have to be carried out in subsequent studies, but now with specialized cells to determine exactly the degree of toxicity depending on the cell origin [24].
| Table 2: Determination of the Mean Lethal Concentration (LC50) for the acute toxicity bioassay with Artemia salina and toxicity with Eisenia foetida. | ||
| Extract | CL50 Artemia sp |
CL50 Eisenia foetida |
| Aqueous | 18412 ppm | 3149.42 ppm |
| Methanol * | N/A | N/A |
| Ethanol | 28012 ppm | N/A |
| Ether | 17706 ppm | 2125.59 ppm |
| Note: N/A stands for Not Available | ||
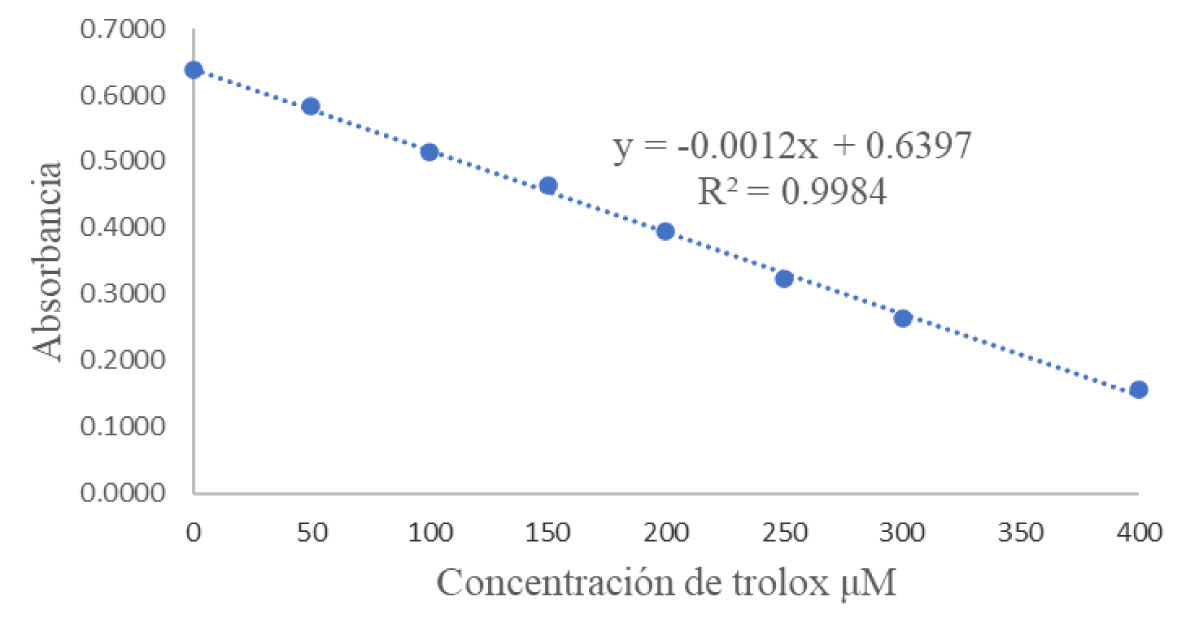
The evaluation of the antioxidant capacity of the Eisenia foetida organisms that were in contact with the extracts of Kalanchoe pinnata proves the relationship of all the results presented above, since the extracts in methanol, ethanol, and ether presented an inhibition percentage of 96%, while the aqueous one 93% (Table 3). More specifically, when looking at the results expressed in mM Trolox (Figure 1. Calibration curve for the ABTS •+ method as generated by measuring the absorbance of the reaction medium at 750 nm. Trolox was used standard), very similar values were determined. However, these values confirm the toxicity of each of them, with ethanol resulting to be the most toxic because of the presence of most of the secondary metabolites, and in a greater proportion, followed by ethanol, methanolic, and finally aqueous.
| : Results of antioxidant evaluation expressed as % inhibition and mM trolox. | ||
| Extract | % inhibition | μM Trolox |
| Aqueous | 93.13 | 498.9167 |
| Methanol | 95.90 | 512.25 |
| Ethanol | 96.11 | 513.9167 |
| Ether | 95.59 | 513.0833 |
Figure 1: Calibration curve of the synthetic antioxidant TROLOX for ABTS.
Most important secondary metabolites were qualitatively identified in the extracts of K. pinnata flowers. Mainly in the alcoholic extracts (methanol and ethanol) tannins, alkaloids, and flavonols were found. As mentioned above, they are reported to have toxicological effects. The toxicity and antioxidant activity tests confirm the preliminary results obtained in the identification of secondary metabolites; concluding that the ethyl extract is the one in which the highest compounds were obtained. In turn, the highest percentage of inhibition was 96.1% and 514 mM of trolox, followed by the methanolic and ethereal extracts with 96% inhibition for each one and 512.25 and 513.08 mM of trolox respectively. However, the toxicity of the ethereal extract may be significantly owed to the kind of solvent used and not so significantly due to the extract, since it did not present a higher amount of secondary metabolites than methyl. Finally, the aqueous extract showed the lowest toxicity, 93% inhibition, and 498.91 mM trolox, finding that these results were not very different from those obtained with the different solvents. It is therefore concluded that the flower of Kalanchoe pinnata contains secondary metabolites that may be of great therapeutic interest.
- Tiwari P, Kumar B, Kaur M, Kaur B, Kaur H. Phytochemical screening and Extraction: A Review. Internationale Pharmaceutica Sciencia. 2011; 1: 99-103.
- Kolodziejczyk-Czepas J, Stochmal A. Bufadienolides of Kalanchoe species: an overview of chemical structure, biological activity and prospects for pharmacological use. Phytochem Rev. 2017;16(6):1155-1171. doi: 10.1007/s11101-017-9525-1. Epub 2017 Aug 2. PMID: 29200987; PMCID: PMC5696496.
- Al- Snafi E. The Chemical Constituents and Pharmacological Effects of Bryophyllum calycinum. A review”. International Journal of Pharma Sciences and Research. 2013; 4: 171-173.
- Sharapin N. Fundamentals of Technology of Phytotherapeutic Products. Ibero-American Program of Science and Technology for Development. First edition. 2000; 158-203.
- Earthworm, Acute Toxicity Test. OECD Guideline for Testing of Chemicals. No. 207. 1984; 1-7.
- González Y, Aportela P. Determination of the Acute Toxicity of Potassium Dichromate in the Larvae of Artemia salina. Center for Toxicology and Biomedicine. 2001; 1(1):104-8. 104-107.
- OECD Guideline for Testinf of Chemicals, “Earthworm, Acute Toxicity Tests”, OECD. 1984; 207: 01-08.
- Schmidt R. Optical motility test for the detection of trichothecenes using brine shrimps. Mycotoxin Res. 1985 Mar;1(1):25-9. doi: 10.1007/BF03191951. PMID: 23605723.
- Törökné A, Vasdinnyei R, Asztalos BM. A rapid microbiotest for the detection of cyanobacterial toxins. Environ Toxicol. 2007 Feb;22(1):64-8. doi: 10.1002/tox.20235. PMID: 17295262.
- McLaughlin JL, Lingling LR, Anderson JE. The use of biological assays to evaluate botanicals. Drug Information J. 1998; 32: 513-524.
- Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Med. 1982 May;45(1):31-4. PMID: 7100305.
- Gutierrez D, Ortega W, Oliveira A, Zaldivar C, Mancini-Filho J. Comparison of the antioxidant properties and polyphenol content of aqueous extracts of the marine algae Bryothamnion triquetrum and Halimeda opuntia. Ars Pharmaceutica. 2015; 56(2): 89-99.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20(7):933-56. doi: 10.1016/0891-5849(95)02227-9. Erratum in: Free Radic Biol Med 1996;21(3):417. PMID: 8743980.
- Reddy MK, Gupta SK, Jacob MR, Khan SI, Ferreira D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007 May;73(5):461-7. doi: 10.1055/s-2007-967167. PMID: 17566148.
- Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000 Dec;52(4):673-751. PMID: 11121513.
- Ganesan S, Faris AN, Comstock AT, Chattoraj SS, Chattoraj A, Burgess JR, Curtis JL, Martinez FJ, Zick S, Hershenson MB, Sajjan US. Quercetin prevents progression of disease in elastase/LPS-exposed mice by negatively regulating MMP expression. Respir Res. 2010 Sep 28;11(1):131. doi: 10.1186/1465-9921-11-131. PMID: 20920189; PMCID: PMC2954923.
- Aziz NH, Farag SE, Mousa LA, Abo-Zaid MA. Comparative antibacterial and antifungal effects of some phenolic compounds. Microbios. 1998;93(374):43-54. PMID: 9670554.
- Robles-García MA, Aguilar AJ, Gutiérrez-Lomelí M, Rodríguez-Félix F, Morales-Del-Río JA. Qualitative identification of secondary metabolites and cytotoxicity determination of tempisque extracts (Sideroxylum capiri pittier). Biotecnia. 2016; XVIII(3): 3-8.
- Maldoni B. Alkaloids: Isolation and purification. Journal of Chemical Education. 1991; 68(8):700-703.
- Waghorn GC, McNabb WC. Consequences of plant phenolic compounds for productivity and health of ruminants. Proc Nutr Soc. 2003 May;62(2):383-92. doi: 10.1079/pns2003245. PMID: 14506885.
- Jean-Blain C. Nutritional and toxicological aspects of tannins. Veterinary Medicine Review. 1988; 149 (10): 911-920.
- Reed JD. Nutritional toxicology of tannins and related polyphenols in forage legumes. Journal of Animal Science. 1995; 73: 1516–1528, https://doi.org/10.2527/1995.7351516x
- Hoste H, Jackson F, Athanasiadou S, Thamsborg SM, Hoskin SO. The effects of tannin-rich plants on parasitic nematodes in ruminants. Trends Parasitol. 2006 Jun;22(6):253-61. doi: 10.1016/j.pt.2006.04.004. Epub 2006 Apr 24. PMID: 16632404.
- Bogucka-Kocka A, Zidorn C, Kasprzycka M, Szymczak G, Szewczyk K. Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoë species. Saudi J Biol Sci. 2018 May;25(4):622-630. doi: 10.1016/j.sjbs.2016.01.037. Epub 2016 Feb 1. PMID: 29740226; PMCID: PMC5936878.