More Information
Submitted: January 18, 2025 | Approved: January 28, 2025 | Published: January 29, 2025
How to cite this article: Serban S, Liu L, Liu Y, Lei X, Zhang C, Li Y, et al. Efficient Sequential Chromatographic Purification of a Recombinant Nanobody-Fc Fusion Designed for Treatment of Severe Fever with Thrombocytopenia Syndrome. Ann Adv Chem. 2025; 9(1): 001-006. Available from:
https://dx.doi.org/10.29328/journal.aac.1001053.
DOI: 10.29328/journal.aac.1001053
Copyright license: © 2025 Serban S, et al. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestriCted use, distribution, and reproduCtion in any medium, provided the original work is properly cited.
Keywords: Nanobody-Fc fusion; Chromatography; SFTS; Affinity chromatography; Protein A
Abbreviations: SFTS: Severe Fever with Thrombocytopenia Syndrome; GN: Glycoprotein N; CIP: Cleaning in Place; DBC: Dynamic Binding Capacity; RT: Retention Time; Fc- Fragment Crystallizable Region of Immunoglobulin; PB: Phosphate Buffer; AFF: Affinity Chromatography; IEC: Ion Exchange Chromatography; HIC: Hydrophobic Interaction Chromatography
Efficient Sequential Chromatographic Purification of a Recombinant Nanobody-Fc Fusion Designed for Treatment of Severe Fever with Thrombocytopenia Syndrome
Simona Serban* , Long Liu, Yan Liu, Xiaoju Lei, Cheng Zhang, Yanjun Li, Xiaokang Kou and Alessandra Basso
, Long Liu, Yan Liu, Xiaoju Lei, Cheng Zhang, Yanjun Li, Xiaokang Kou and Alessandra Basso
Sunresin New Materials Co Ltd., 135 Jinye Rd., Xi’an, Shaanxi 710076, China
*Address for Correspondence: Simona Serban, Sunresin New Materials Co Ltd., 135 Jinye Rd., Xi’an, Shaanxi 710076, China, Email: [email protected]
Severe fever with thrombocytopenia syndrome (SFTS) is caused by a virus that induces acute infections. Despite its expansion beyond China, where it first appeared in 2009, no specific drug exists to treat the disease. The discovery that antibodies targeting the SFTS virus surface glycoprotein (Glycoprotein N, GN) significantly enhance patient survival has driven the development of antibodies, particularly nanobodies. Nanobodies targeting the GN protein are a promising therapeutic approach. This paper presents a systematic study of the purification process for a recombinant nanobody-Fc fusion designed to treat the SFTS virus HB29. The study evaluated a sequential purification approach using affinity (AFF), ion exchange (IEC), and hydrophobic interaction chromatography (HIC) techniques to gradually remove impurities. The results demonstrate that this approach achieves an overall yield of more than 50% and a total purity of 95%. Efficient nanobody purification methods, as outlined here, can pave the way for novel treatments to manage this disease.
Severe fever with thrombocytopenia syndrome (SFTS) is an acute infectious disease caused by a virus primarily transmitted through insect bites by Asian longhorned tick (Haemaphysal is longicornis (H. longicornis) or contact with the blood or mucous membranes of infected patients [1]. Since its first appearance in China in 2009, the disease has spread to South Korea, Japan, Saudi Arabia, and the United States [2].
To date, there is no specific drug available for the treatment of SFTS. However, specific antibodies have demonstrated good clinical efficacy in antiviral treatments, such as the respiratory syncytial virus monoclonal antibody Palivizumab and rabies virus antiserum. Studies have shown that antibodies targeting the SFTS virus surface Glycoprotein N (GN) play a critical role in patient survival. Consequently, significant efforts are being made to design antibodies targeting GN as effective therapies to combat the SFTS virus [2,3].
Recently, an SFTS-neutralizing nanobody against GN was reported, which can be utilized either as a screening kit or as a drug [4].
The discovery of nanobodies dates back to 1993 when a new type of natural antibody derived from the camelid family was identified. Nanobodies naturally lack light chains and consist solely of heavy chains. The relative molecular mass of a nanobody is approximately 15 kDa, making it about ten times lighter than a typical antibody [5].
Due to their high stability, strong affinity, more than 80% homology with human antibodies, low toxicity, and minimal immunogenicity, nanobodies have been extensively used in immunodiagnostic kits [6], imaging research and development, and antibody drug development in the fields of tumors [7], inflammation, infectious diseases, and nervous system diseases [5,8,9].
However, efficient purification technologies for nanobodies are still in the development phase. Their smaller size and unique conformation necessitate a novel approach to purification strategies [10,11].
In this paper, we present a sequential purification strategy for a recombinant nanobody-Fc fusion antibody targeting the treatment of SFTS [12,13]. The nanobody combines its epitope-binding properties with the detection capabilities of Fc-domains, enabling the use of affinity resins for efficient purification.
All reagents were of analytical grade and were purchased from Sinopharm Chemical Reagent Co. Ltd, China and all solutions were made with ultrapure water. The chromatographic experiments were performed using the GE AKTA pure chromatography system, Cytiva, China, and the SDS-PAGE was performed using Protein Electrophoresis Equipment from Bio-Rad, China. SDS-PAGE experiments have been performed both in native and reduced form using 2-mercaptoethanol.
The chromatography resins selected for the sequential purification processes were: rProteinA Seplife Suno (AFF), Seplife Phenyl Large Scale HP (HIC), Seplife 30HS (30HS), Seplife 50HQ (50HQ), and Seplife 50HS (50HS) all manufactured at Sunresin New Materials Co. Ltd. (Table 1) and prepacked in chromatography columns 0.8 x 10 cm size, 5 ml volume.
All the purification steps were based on binding the nanobody with a 5-minute contact time. Different sample volumes were used for different steps depending on the sample concentration and the dynamic binding capacity of the resin. Details about the sequential chromatographic process, as well as the binding and elution buffers used in the process, are described in Table 2. The contact time for the different steps was adjusted to maximize process efficiency, and the cleaning in place (CIP) was performed with 0.1 M NaOH. The AFF step remained consistent across all three purification protocols.
The inhibition rate against SFTS HB29 was performed using an immunoassay SFTS kit provided by Yuandaolong (Suzhou) Medical Technology Co. Ltd., China.
The recombinant nanobody-Fc fusion purified has a molecular weight of 65 kDa, an isoelectric point of 6.2, and was produced by fermentation in an eukaryotic microorganism. A sequential chromatography approach comprising three distinct steps was considered for the downstream purification process. Given that the nanobody-Fc fusion contains an Fc-tag, the first initial step to apply was an affinity chromatography step based on rProtein A Seplife Suno [14,15]. The initial AFF step provides a rapid capture of the recombinant nanobody and an efficient means of obtaining the molecule of interest with a purity > 70%. The second and third steps are designed to further remove aggregates [16], endotoxins [17], and host cell proteins [18] remaining after the initial capture step. Following this approach, in our sequential approach, we have included a hydrophobic-based purification (HIC). In fact, HIC presents notable benefits in aggregate removal, owing to the increased surface hydrophobicity exhibited by aggregates in comparison to monomers [19]. Since nanobodies belong to that class of proteins that cannot be separated effectively by using chromatography with a single mechanism, we also evaluated the effect of charge-based purification. Aggregation gives rise to protein surface coverage, leading to disparities in surface charge when compared to individual monomers, and aggregates are usually more tightly bound to the cation exchange resin than monomeric proteins. On the other side, endotoxins are usually eliminated in the flow-through when using a strong acid cation resin and will bind to a strong base anion resin, so we have also included an ion exchange step.
Each purification step is carefully designed to increase the purity of the molecule of interest without compromising the overall yield. The sequential chromatographic purification described herein aims to achieve a final purity of ≥ 95% (determined by the SE-HPLC test) and the highest possible overall yield, > 50%.
The chromatography resins selected for the second and third purification steps were Seplife Phenyl Large Scale HP (HIC), Seplife 30HS (30HS), Seplife 50HQ (50HQ), and Seplife 50HS (50HS). These resins were used in various combinations, recognizing that the type of chromatographic interaction and their order significantly impact process performance. The details of the chromatography resins used in this work are presented in Table 1.
| Table 1: Characteristics of the chromatographic resins employed for the differentpurification case studies. | |||
| Chromatographicresin | Type and functionalgroup | Particle size range(microns) | Polymer matrix |
| rProteinA SeplifeSuno | AFF – Protein A,Affinity to Fc region | 40-100 (mean diameter 70) |
Agarose (4%) |
| Seplife Phenyl LargeScale HP | HIC - Hydrophobicinteraction | 30-50 (mean diameter 37) |
Agarose (6%) |
| Seplife LXMS 50HQ | IEC – Quaternaryamine | 40-60 (mean diameter 50) |
Polystyrene/divinylbenzene |
| Seplife LXMS 50HS | IEC – Suphopropyl | 40-60 (mean diameter 50) |
Polystyrene/divinylbenzene |
| Seplife LXMS 30HS | IEC – Suphopropyl | 27-33 (mean diameter 30) |
Polystyrene/divinylbenzene |
| Abbreviations: AFF:Affinity Purification using rProteinA Seplife®Suno resin; HIC: Hydrophobic Interaction Chromatography; IEC: Ion ExchangeChromatography | |||
The target was to evaluate the different nanobody-resin interactions with the aim of gradually eliminating impurities that have different or similar properties to the molecule of interest. Besides the affinity capture by the protein A resin, other interactions explored included ionic binding (IEC) to the resin, either at a pH higher or lower than the isoelectric point of the recombinant nanobody, and hydrophobic interactions with phenyl functional groups on the HIC resin. During the resin screening process, multimodal and other hydrophobic interactions were also tested, but the results were poor (data not shown).
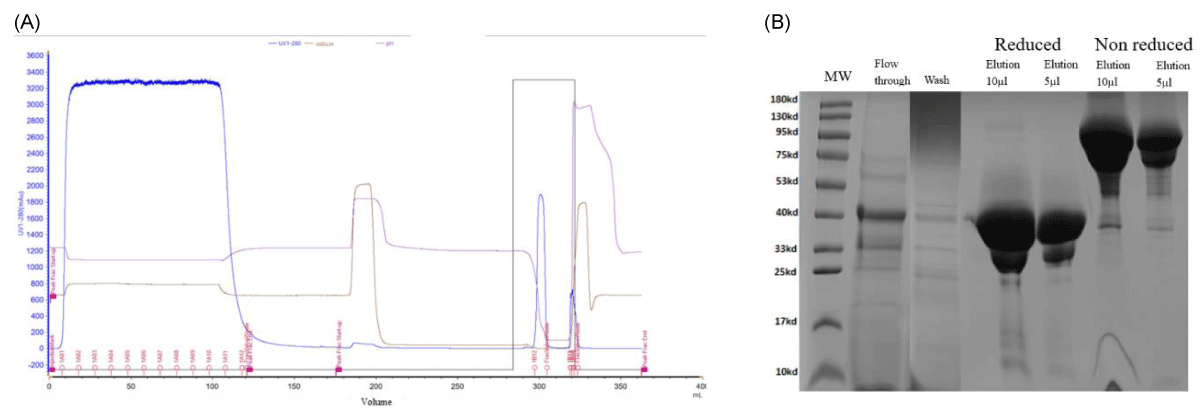
Affinity purification using rProteinA Seplife Suno is the key step in the purification process, and the chromatogram illustrating this step is presented in Figure 1A. The AFF Suno resin was independently designed and manufactured at Sunresin and exhibits a high 10% dynamic binding capacity (DBC) (> 70 mg/mL IgG at 5 min retention time [RT]) and exceptional alkaline stability during cleaning-in-place (CIP) with 1 M NaOH. The resin maintains over 85% DBC after 100 CIP cycles [15].
Figure 1: (A) Chromatogram illustrating the affinity purification of the molecule on the rProteinA Seplife Suno. 100 ml feed at pH 6.3 and conductivity 40 mS/cm were loaded (retention time 5 min), with a 10ml elution volume. (B) SDS-PAGE recorded using sample fractions from the affinity purification on rProteinA Seplife® Suno.
The fractions collected during the affinity purification process have been tested by SDS-PAGE using reduced and non-reduced methods and are shown in Figure 1B. The non-reduced sample shows the molecule at 80 and 130kDa in the quaternary structure while the reduced sample was treated with a reducing agent to break the disulfide bonds formed between cysteine amino acids and shows only the main subunit of the recombinant nanobody at 40kDa.
The initial fermentation broth sample had a recombinant nanobody purity of 40% which was increased to 70.5% after the AFF capture step at a yield of 95%.
The elution sample collected during the AFF step was diluted and pH adjusted as per Table 2 to suit the next purification step.
| Table 2: Chromatographic conditions for the three purification protocols each using three steps. | |||
| Chromatographic step | Sample buffer | Wash buffer | Elution buffer |
| Purification protocol 1 | |||
| AFF | 20 mM PB, 0.35 M NaCl, pH 7.0 | 50 mM Tris-HCl, 0.25 M Arg HCl, 1 M NaCl, pH 9.0 And 20 mM PB, pH 7.0 |
20 mM citrate buffer, pH 3.2 |
| HIC | Sample from affinity elution, conductivity adjusted to 80 mS/cm, pH 7.0 | 20 mM PB, 0.5 M ammonium sulphate, pH 7.0 | 20 mM PB, pH 7.0 |
| IEC with strong acid cation (30HS) | Sample from HIC elution pH adjusted to 5 with diluted acetic acid | 50 mM acetate buffer, pH 5.0 | 50 mM acetate buffer, 1M NaCl, pH 5.0 |
| Purification protocol 2 | |||
| AFF | 20 mM PB, 0.35 M NaCl, pH 7.0 | 50 mM Tris-HCl, 0.25 M Arg HCl, 1 M NaCl, pH 9.0 And 20 mM PB, pH 7.0 |
20 mM citrate buffer, pH 3.2 |
| IEC with strong base anion (50HQ) | Sample from affinity elution, pH adjusted to 8.5 using TRIS buffer | 50 mM Tris-HCl, pH 8.5 | 50 mM Tris-HCl, 1 M NaCl, pH 8.5 |
| IEC with strong acid cation (50HS) | Sample from previous elution, diluted to conductivity 7mS/cm, pH adjusted to 5.0 with acetic acid | 50 mM acetate buffer, pH 5.0 | 50 mM acetate buffer , 1 M NaCl, pH 5.0 |
| Purification protocol 3 | |||
| AFF | 20 mM PB, 0.35 M NaCl, pH 7.0 | 50 mM Tris-HCl, 0.25 M Arg HCl, 1 M NaCl, pH 9.0 And 20 mM PB, pH 7.0 |
20 mM citrate buffer, pH 3.2 |
| IEC with strong base anion (50HQ) | Sample from affinity elution, pH adjusted to 8.5 using TRIS buffer | 50 mM Tris-HCl, pH 8.5 | 50 mM Tris-HCl, 0.3 M NaCl, pH 8.5 |
| HIC | Sample from IEC elution, conductivity adjusted to 80mS/cm, pH 7 | 50 mM Tris-HCl, 0.5 M ammonium sulphate, pH 8.5; and 50 mM Tris-HCl, 0.35 M ammonium sulphate, pH 8.5 |
0.2 M Tris-HCl, pH 8.5 |
| Abbreviations: AFF: Affinity Purification using rProteinA Seplife® Suno resin; HIC: Hydrophobic Interaction Chromatography; IEC: Ion Exchange Chromatography. | |||
Although all the purification steps described in Table 2 are of interest, we are presenting here only the chromatographic data corresponding to the last purification process using AFF followed by IEC – 50HQ and then the last HIC step which gave the best results. The summary of all three sequential purification processes is presented in Table 3 indicating that purification protocol 3 is the most successful.
| Table 2: Chromatographic conditions for the three purification protocols each using three steps. | |||
| Chromatographic step | Sample buffer | Wash buffer | Elution buffer |
| Purification protocol 1 | |||
| AFF | 20 mM PB, 0.35 M NaCl, pH 7.0 | 50 mM Tris-HCl, 0.25 M Arg HCl, 1 M NaCl, pH 9.0 And 20 mM PB, pH 7.0 |
20 mM citrate buffer, pH 3.2 |
| HIC | Sample from affinity elution, conductivity adjusted to 80 mS/cm, pH 7.0 | 20 mM PB, 0.5 M ammonium sulphate, pH 7.0 | 20 mM PB, pH 7.0 |
| IEC with strong acid cation (30HS) | Sample from HIC elution pH adjusted to 5 with diluted acetic acid | 50 mM acetate buffer, pH 5.0 | 50 mM acetate buffer, 1M NaCl, pH 5.0 |
| Purification protocol 2 | |||
| AFF | 20 mM PB, 0.35 M NaCl, pH 7.0 | 50 mM Tris-HCl, 0.25 M Arg HCl, 1 M NaCl, pH 9.0 And 20 mM PB, pH 7.0 |
20 mM citrate buffer, pH 3.2 |
| IEC with strong base anion (50HQ) | Sample from affinity elution, pH adjusted to 8.5 using TRIS buffer | 50 mM Tris-HCl, pH 8.5 | 50 mM Tris-HCl, 1 M NaCl, pH 8.5 |
| IEC with strong acid cation (50HS) | Sample from previous elution, diluted to conductivity 7mS/cm, pH adjusted to 5.0 with acetic acid | 50 mM acetate buffer, pH 5.0 | 50 mM acetate buffer , 1 M NaCl, pH 5.0 |
| Purification protocol 3 | |||
| AFF | 20 mM PB, 0.35 M NaCl, pH 7.0 | 50 mM Tris-HCl, 0.25 M Arg HCl, 1 M NaCl, pH 9.0 And 20 mM PB, pH 7.0 |
20 mM citrate buffer, pH 3.2 |
| IEC with strong base anion (50HQ) | Sample from affinity elution, pH adjusted to 8.5 using TRIS buffer | 50 mM Tris-HCl, pH 8.5 | 50 mM Tris-HCl, 0.3 M NaCl, pH 8.5 |
| HIC | Sample from IEC elution, conductivity adjusted to 80mS/cm, pH 7 | 50 mM Tris-HCl, 0.5 M ammonium sulphate, pH 8.5; and 50 mM Tris-HCl, 0.35 M ammonium sulphate, pH 8.5 |
0.2 M Tris-HCl, pH 8.5 |
| Abbreviations: AFF: Affinity Purification using rProteinA Seplife® Suno resin; HIC: Hydrophobic Interaction Chromatography; IEC: Ion Exchange Chromatography. | |||
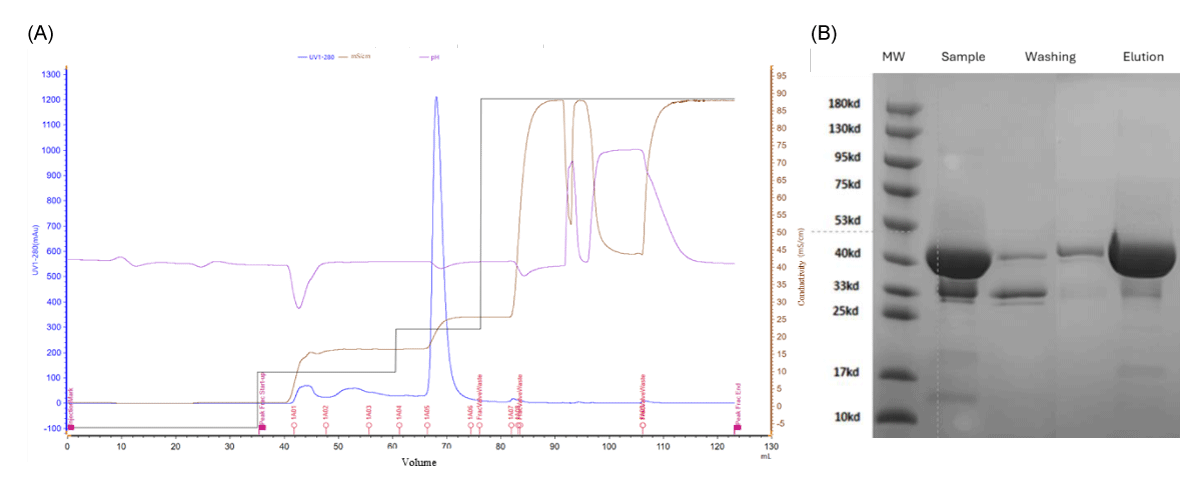
In the purification protocol 3, the recombinant nanobody purity after the IEC step with Seplife LXMS 50HQ increased from 70.5% to 90.2% and the yield of the step was 85%; Figure 2A represents the elution conditions applied in this purification step. This chromatographic step mostly removes the smaller molecular weight proteins that are HCP products of the fermentation as indicated in Figure 2B.
Figure 2: (A) - Chromatogram illustrating the IEC step with Seplife® LXMS 50HQ. 15 ml of the AFF Suno elution was pH adjusted to 8.5 and injected in the second column at a retention time 5 min. Elution was done in a step manner increasing the NaCl concentration to 17% for a first wash step and to 30% to elute the recombinant nanobody (10 ml elution fraction). (B) SDS-PAGE reduced conditions to show the purity of the different fractions.
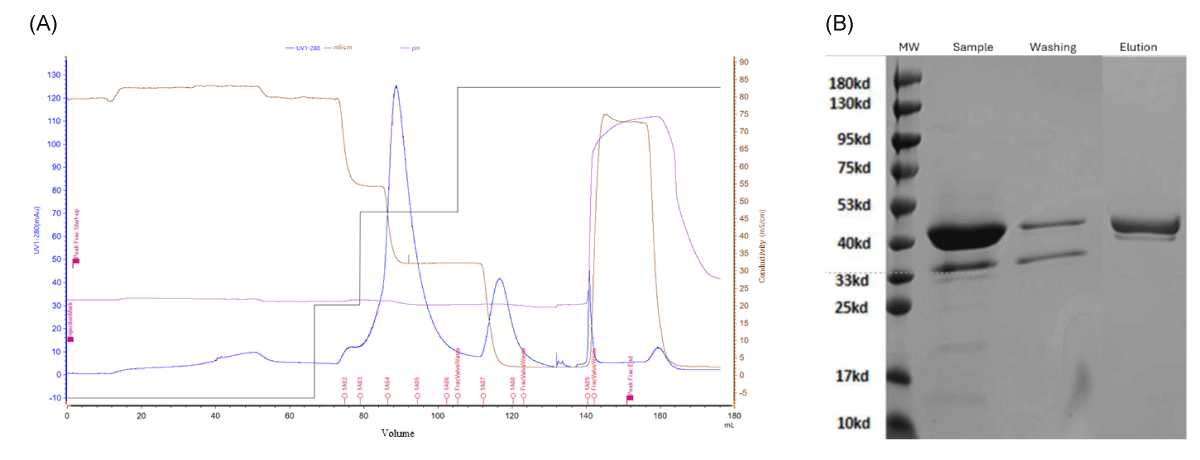
The third purification step of Purification Protocol 3 is the HIC step, which uses Seplife Phenyl Large Scale HP. The elution fraction from the previous IEC-50HQ step was adjusted to pH 7.0 and increased in conductivity using ammonium sulfate to promote hydrophobic interaction between the nanobody and the resin (Table 2). The HIC purification profile is presented in Figure 3A indicating the conductivity, pH, and overall elution conditions.
As shown in Figure 3B, the HIC step further removed small molecular weight HCP contaminants, increasing the purity of the elution fraction to approximately 95%. The yield of the chromatography step was 65%, primarily due to some sample loss during the wash step.
Figure 3: (A) - Chromatogram showing the elution profile of the recombinant nanobody purification by HIC using Seplife® Phenyl Large Scale HP. The elution fractions from the previous IEC step have been conductivity and pH adjusted and loaded on the column at 5 minutes retention time. A wash step at 0.35 M ammonium sulphate in PB and elution step with 0.2 M ammonium sulphate in PB pH 7.0 were applied. The elution fraction of 23 ml was collected representing the final product. (B) Reduced SDS-PAGE indicating the purity of different elution fractions.
The difference in yield between the various purification processes (Table 3) is mainly related to the interaction strength between the recombinant nanobody and the chromatographic resin. For instance, the difference between Protocols 2 and 3 lies in the final purification step, which results in a significant yield difference of approximately 40%. This occurs because a portion of the product is lost during the washing steps and does not efficiently or fully elute from the 50HS resin, instead being recovered in the CIP fraction. Similar issues with product loss in the wash and CIP fractions were observed for purification protocol 1, contributing to its low yield. Additionally, endotoxins can be retained by both the IEC-50HQ and HIC chromatography steps, resulting in a purer final product.
| Table 3: Overall results for the three sequential purification processes. | |||
| Process route | Yield | Purity | Advantages and Disadvantages |
| Protocol 1: AFF - HIC - 30HS | 20% - 30% | > 95% | High purity, low yield |
| Protocol 2: AFF - 50HQ - 50HS | 10% - 20% | > 95% | Very low yield, high purity, slightly higher activity |
| Protocol 3: AFF - 50HQ - HIC | 50% - 60% | > 95% | High yield, slightly lower purity |
| Abbreviations: AFF: Affinity Purification using rProteinA Seplife® Suno resin; HIC: Hydrophobic Interaction Chromatography; 50HQ: Strong Base Anion Resin Seplife LXMS 50HQ. | |||
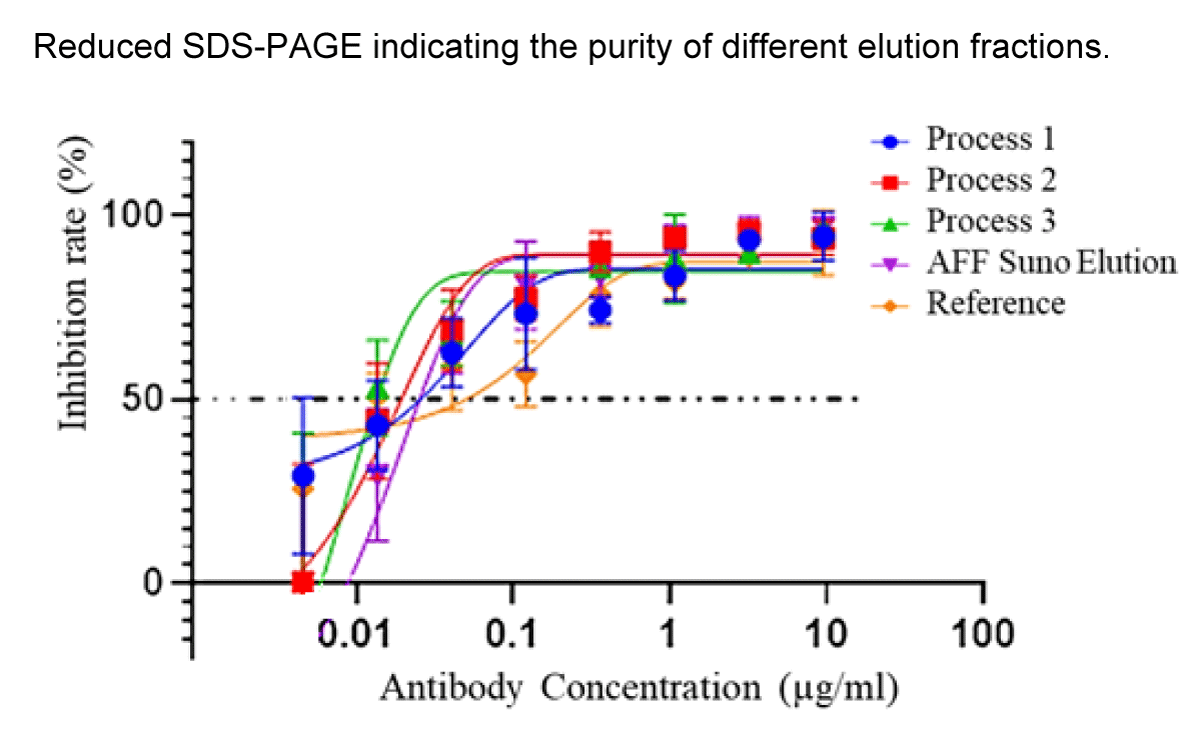
The results from Figure 4 show that the different purification routes produce molecules with similar activity in terms of virus inhibition, making the differentiating factor the process yield. This confirms that purification protocol 3 is suitable for producing the desired antibody molecule with an effective inhibitory effect on the HB29 virus and the highest yield.
Figure 4: Recombinant nanobody samples obtained from the different purification processes and from the AFF elution were compared in terms of inhibition rate against SFTS HB29 virus showing similar results.
The variety of interactions between the nanobody-Fc fusion molecule and the chromatography media described in this paper showed that with careful selection and adjustment of the operating condition, a purity of 95% was obtained with suitable potency for the SFTS HB 29 virus inhibition. Further work such as a further purification step (SEC) may be considered to increase the molecule purity to 99.7% or even changes in the upstream process may be beneficial to increase the yield and purity of the downstream process.
We have presented the development of a procedure for the purification of a recombinant nanobody-Fc fusion designed for the treatment of SFTS virus HB29. Nanobodies efficiently target the SFTS virus surface glycoprotein N, which plays a critical role in patient survival, and they remain the most promising approach for treating this disease.
In this paper, we developed and compared three sequential purification technologies based on affinity, ion exchange, and hydrophobic interaction. The goal of the work was to evaluate the interactions between the nanobody and the resin, with the aim of gradually eliminating impurities with varying properties relative to the molecule of interest. The study demonstrated that using a sequential process comprising affinity purification with protein A resin, followed by ion exchange and an HIC step, it is possible to improve yield from 30% to 60% and achieve an increased total purity from 40% to 95%. Of course, this approach needs to be adapted to the different protein sources. Screening of chromatography techniques is important and represents a key step in the development of a suitable manufacturing process. The advancements in robotics and fast screening technologies make this process faster and more efficient.
- Casel MA, Park SJ, Choi YK. Severe fever with thrombocytopenia syndrome virus: emerging novel phlebovirus and their control strategy. Exp Mol Med. 2021;53:713–722. Available from: https://doi.org/10.1038/s12276-021-00610-1
- Ren X, Sun J, Kuang W, Yu F, Wang B, Wang Y, et al. A broadly protective antibody targeting glycoprotein Gn inhibits severe fever with thrombocytopenia syndrome virus infection. Nat Commun. 2024;15:7009–7023. Available from: https://www.nature.com/articles/s41467-024-51108-z
- Kim KH, Kim J, Ko M, Chun JY, Kim H, Kim S, et al. An anti-Gn glycoprotein antibody from a convalescent patient potently inhibits the infection of severe fever with thrombocytopenia syndrome virus. PLOS Pathog. 2019;21:1–21. Available from: https://doi.org/10.1371/journal.ppat.1007375
- Xilin W, Zhiwei W, Yanlei L, Yi P. A SFTSV detection kit. CN 110684102A. 2019.
- Liu M, Li L, Jin D, Liu Y. Nanobody—A versatile tool for cancer diagnosis and therapeutics. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13:e1697. Available from: https://doi.org/10.1002/wnan.1697
- Salvador JP, Vilaplana L, Marco MP. Nanobody: outstanding features for diagnostic and therapeutic applications. Anal Bioanal Chem. 2019;411:1703–1713. Available from: https://doi.org/10.1007/s00216-019-01633-4
- Bannas P, Hambach J, Koch-Nolte F. Nanobodies and Nanobody-Based Human Heavy Chain Antibodies As Antitumor Therapeutics. Front Immunol. 2017;8:1603. Available from: https://doi.org/10.3389/fimmu.2017.01603
- Jovčevska I, Muyldermans S. The Therapeutic Potential of Nanobodies. BioDrugs. 2020;34:11–26. Available from: https://doi.org/10.1007/s40259-019-00392-z
- Jin B, Odongo S, Radwanska M, Magez S. NANOBODIES: A Review of Generation, Diagnostics and Therapeutics. Int J Mol Sci. 2023;24:5994. Available from: https://doi.org/10.3390/ijms24065994
- Stevens TA, Tomaleri GP, Hazu M, Wei S, Nguyen VN, et al. A nanobody-based strategy for rapid and scalable purification of human protein complexes. Nat Protoc. 2024;19:127–158. Available from: https://doi.org/10.1038/s41596-023-00904-w
- Haddad M, Soukkarieh C, Khalaf HE, Abbady AQ. Purification of polyclonal IgG specific for Camelid’s antibodies and their recombinant Nanobodies. Open Life Sci. 2016;11:1–9. Available from: https://doi.org/10.1515/biol-2016-0001
- Liu Q, Lu Y, Cai C, Huang Y, Zhou L, Guan Y, et al. A broad neutralizing nanobody against SARS-CoV-2 engineered from an approved drug. Cell Death Dis. 2024;15:458. Available from: https://www.nature.com/articles/s41419-024-06802-7
- Ji M, Hu J, Zhang D, Huang B, Xu S, Jiang N, et al. Inhibition of SFTSV replication in humanized mice by a subcutaneously administered anti-PD1 nanobody. EMBO Mol Med. 2024;16:575–595. Available from: https://doi.org/10.1038/s44321-024-00026-0
- Basso A, Serban S, Gu TN, Liu L, Li YJ. Innovation in mAb purification using affinity chromatography resins based on proprietary rProtein A. TWENTYFOURSEVENBIOPHARMA. 2024;1:66–68. Available from: https://247biopharma.com/article/innovation-in-mab-purification-using-affinity-chromatography-resins-based-on-proprietary-rprotein-a/
- Yanjun L, Gang L, Tongnian G, Jiantao Z, Long L, Xiaoju L, et al. Polypeptide, fusion type polymer protein and application thereof. CN115850408A. 2022.
- Chen T, Guo G, Tan G, Wang Y, Li Y. Antibody aggregate removal using a mixed-mode chromatography resin. Methods Mol Biol. 2021;2178:345–354. Available from: https://doi.org/10.1007/978-1-0716-0775-6_23
- Sakata M, Yamaguchi Y. Affinity chromatography removes endotoxins. BioPharm Int. 2005;18. Available from: https://www.biopharminternational.com/view/affinity-chormatography-removes-endotoxins
- Li Y. Effective strategies for host cell protein clearance in downstream processing of monoclonal antibodies and Fc-fusion proteins. Protein Expr Purif. 2017;134:96–103. Available from: https://doi.org/10.1016/j.pep.2017.04.006
- Tang S, Tao J, Li Y. Challenges and solutions for the downstream purification of therapeutic proteins. Antibody Ther. 2024;7:1–12. Available from: https://doi.org/10.1093/abt/tbad028